0051
The dynamic BOLD-CBF coupling during resting-state in the aging brain: a dual-echo pCASL study1Neuroscience, Imaging and Clinical Sciences, Department of Neuroscience, Imaging and Clinical Sciences - Institute for Advanced Biomedical Technologies, Chieti, Italy
Synopsis
Simultaneous acquisition of BOLD and CBF data using ASL allows the study of resting-state brain function from a different perspective with respect to functional connectivity. In particular, recent evidence showed that spontaneous BOLD and CBF fluctuations are strongly coupled in the cortex. Here we show that this coupling is markedly reduced in elderlies with normal cognitive scores, with a regional specific effect in the left supramarginal gyrus, an area known to be involved in different memory functions. These findings suggest that the study of dynamic BOLD-CBF coupling can potentially offer early biomarkers of functional changes in the aging brain.
INTRODUCTION
FMRI based on arterial spin labeling (ASL) has recently attracted increasing interest because it can extend the study of resting-state brain function beyond that of functional connectivity, allowing quantitative cerebral blood flow (CBF) measurements and the investigation of BOLD-CBF coupling when the two signals are acquired simultaneously1. In particular, a recent study showed a significant synchronization between spontaneous BOLD and CBF fluctuations throughout the cortex, and especially within the major nodes of established resting-state networks2. In the present study we investigated how the dynamic BOLD-CBF coupling during resting-state is affected by aging.METHODS
Fifteen young subjects (mean age=26.4, SD=4.2) and 17 healthy elderlies (mean age=63.4, SD=8.1) were studied. Elderlies were screened with a battery of tests including Mini Mental State Examination (score>25.5), Babcock story test (score>5.1) and the Frontal Assessment Battery (score>14.7) to evaluate global cognitive, memory and executive functions. FMRI was performed with a 3T Achieva Philips scanner using a whole-body radiofrequency coil for signal excitation and an 8-channel phased-array head coil for signal reception. Resting-state CBF and BOLD data were simultaneously acquired with a dual-echo pseudo-continuous ASL (pCASL) sequence (matrix 64x64, voxel size 3.6mm x 3.6mm x 5mm, SENSE factor 2.3, TE1/TE2 10/28 ms, TR 3.5s, 19 slices, time frames 90, labeling duration 1.5s, post-labeling delay 1s). Cardiac (ppu) and respiratory (belt) data were also acquired. Image analysis was performed using AFNI. The first preprocessing steps were performed on the label and control ASL images separately, including RETROICOR to remove signal fluctuations related to cardiac and respiratory cycles, motion correction to realign all time frames and ANATICOR to remove nuisance regressors derived from the six motion parameters, large ventricles and local white matter regions3. To reduce BOLD contamination, CBF timeseries were derived from short TE data by high-pass filtering the ASL signal (cutoff 0.071 Hz) and then demodulating it through multiplication by cos[π(n-1)], where n is the frame number4. BOLD timeseries were estimated by surround addition of the label and control signals acquired at the long TE. Preprocessed functional scans were then spatially normalized using Advanced Normalization Tools5. With a final warp of the study specific template to the MNI standard space. Finally, BOLD and CBF data were spatial smoothed (6mm FWHM) and band-pass filtered (0.01-0.071 Hz). Correlation (Pearson r) between BOLD and CBF timeseries was calculated for each voxel to obtain individual maps representing the BOLD-CBF coupling during resting-state. This calculation was performed considering both a zero-lag between the two signals (r0) and the lag yielding the maximum correlation value (rmax, lag range -7s ¸ 7s) to evaluate the impact of age-dependent hemodynamic time-shifts on the BOLD-CBF coupling. Random effects group maps (one sample t-test) were then obtained after individual r0 and rmax to z-Fisher transform for young and elderly separately and then compared between groups (two sample unpaired t-test). Group maps and contrasts were thresholded at p<0.05, FDR corrected.RESULTS
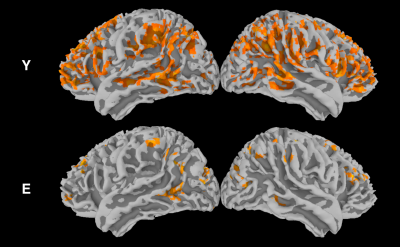
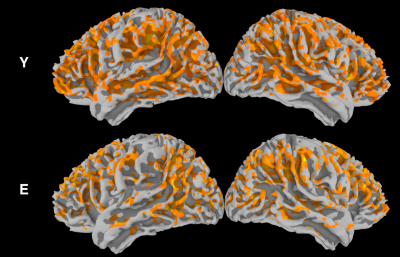
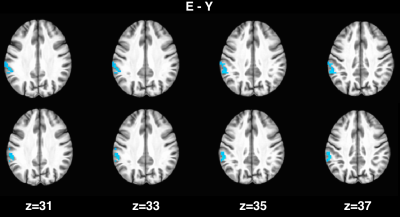
For young subjects, the group analysis (Fig. 1) showed a significant correlation between spontaneous fluctuations of BOLD and CBF signals, especially in frontal and parietal areas corresponding to tha default mode and frontoparietal networks. In elderlies, this correlation was markedly reduced. When applying time-shift correction, the correlation increased in both groups but with a larger effect for elderlies (Fig. 2). However, the between-group contrast showed a significant reduced BOLD-CBF correlation in elderlies in the left supramarginal gyrus with both r0 and rmax analysis (Fig. 3). Moreover, a control analysis measuring the amplitude of spontaneous fluctuations (RSFA)6 revealed no significant between-group differences for both BOLD and CBF.DISCUSSION
As a new finding, we observed that the correlation between spontaneous BOLD and CBF fluctuations is significantly reduced in elderly individuals, especially in the left supramarginal gyrus, an area known to be involved in verbal working memory and episodic memory. Correcting for temporal shift between BOLD and CBF timecourses resulted in an increased correlation for both groups, but with a larger increase for elderlies. However, even after temporal shift correction, a significant between-group difference was still observed in the left supramarginal gyrus, indicating that the age related dynamic BOLD-CBF uncoupling in this region is more pronounced and can be only partially explained with a simple time-shift between the two signals.CONCLUSION
The present results are particularly interesting, considering that the included elderlies showed normal cognitive functions. Indeed, the observed differences suggest that the study of dynamic coupling between BOLD and CBF spontaneous fluctuations could potentially provide early biomarkers of functional changes in the aging brain. Further investigation should address the physiological underpinnings of the observed results.Acknowledgements
No acknowledgement found.References
1. Chen JJ, Jann K, Wang DJJ. Characterizing Resting-State Brain Function Using Arterial Spin Labeling. Brain Connectivity. 2015;5:527–542.
2. Tak S, Wang DJJ, Polimeni JR, et al. Dynamic and static contributions of the cerebrovasculature to the resting-state BOLD signal. NeuroImage. 2014;84:672–680.
3. Jo HJ, Saad ZS, Simmons WK, et al. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. NeuroImage. 2010;52:571–582.
4. Chuang K-H, van Gelderen P, Merkle H, et al. Mapping resting-state functional connectivity using perfusion MRI. NeuroImage. 2008;40:1595–1605.
5. Avants BB, Cook P, Pluta J, et al. Multivariate Diffeomorphic Analysis of Longitudinal Increase in White Matter Directionality and Decrease in Cortical Thickness between Ages 14 and 18. NeuroImage. 2009;47:S105.
6. Kannurpatti SS, Biswal BB. Detection and scaling of task-induced fMRI-BOLD response using resting state fluctuations. NeuroImage. 2008;40:1567–1574.
Figures