0035
Simultaneous Proton Density Fat Fraction Imaging and Water T1-Mapping with Low B1+ Sensitivity (PDFF-T1)Richard B Thompson1, Kelvin Chow2, and Justin Grenier1
1Department of Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Cardiovascular MR R&D, Siemens Medical Solutions USA, Inc., Chicago, IL, United States
Synopsis
Quantitative multi-parametric MR imaging is an important component of diagnosis and objective staging of diffuse liver disease, reducing the need for biopsy. The goal of the current study was to validate a new simultaneous proton density fat fraction and water T1-mapping approach (PDFF-T1) with low B1+ sensitivity in a short, patient-friendly breath-hold acquisition. It is shown (simulations, phantoms, in-vivo) that a time-varying flip angle excitation scheme leads to improved accuracy of PDFF and that fat-water separation enables water-specific T1 mapping, all in a single patient-friendly breath-hold, with low sensitivity to inhomogeneity in the transmitted radiofrequency (B1+) field.
INTRODUCTION
Quantitative multi-parametric MR imaging is an important component of diagnosis and objective staging of diffuse liver disease, cirrhosis and other precursor diseases, reducing the need for biopsy. (1) Imaging of fat content and tissue fibrosis (via T1 quantification) in particular provide complimentary information. Currently, fat fraction and T1 mapping require separate acquisitions, potentially with an additional separate radio-frequency transmit (B1+) field map to account for excitation inhomogeneity. The goal of the current study was to validate a new simultaneous proton density fat fraction and water T1-mapping approach (PDFF-T1) with low B1+ sensitivity in a short, patient-friendly breath-hold acquisition.METHODS
To achieve the simultaneous goals of fat fraction quantification, water T1 quantification and B1+ insensitivity, an investigational prototype saturation-recovery multi-echo gradient-echo Dixon approach with ramped excitation flip angles is proposed. Non-saturation and saturation recovery images were acquired sequentially (Fig. 1). T1 was evaluated from Dixon water-separated images using signal ratios (lookup table approach based on Bloch equation simulations, including actual slice profile). Bloch equation simulations were also used to evaluate the dependence of calculated T1 on B1+ for comparison of conventional constant flip angle and ramped-flip-angle approaches. Fat fraction was evaluated from non-saturation images (conventional Dixon approach (2)). Pulse Sequence Parameters: 3 interleaved slices, 8 mm thickness, 192x144 acquisition matrix, 400 x 333 mm2 FOV, GRAPPA = 2, 1600 Hz/pixel receiver bandwidth, TR = 36ms, 6 equally spaced TEs 1.23 – 10.23 ms, flip angle = 20°, one non-sat image and four saturation recovery images (TS=1302ms), 13 second acquisition time. Ramped flip angles followed a sin(q) pattern with increasing RF pulse index, with maximum value (q=p/2) at the last RF pulse.(3) A custom 6-pulse saturation train was used to ensure >99% saturation efficiency at 3T. (3) Phantom and in-vivo experiments were completed on a 3T scanner (MAGNETOM Prisma, Siemens Healthcare Erlangen, Germany). NiCl2-agarose phantoms with a wide range of T1 and T2 values were used for validation of the T1 mapping approach (comparison to saturation-recovery spin-echo experiments). Fat fraction and water T1 images from the proposed PDFF-T1 approach were acquired from a healthy control and subject with mild fatty liver disease.RESULTS
Figure 2 compares the simulated signal yield of the constant and ramped-flip-angle approach as a function of T1, for the target flip angle of 20°. The constant-flip-angle readout yields ~50% signal variation over the range of T1 values (300-2000ms) while the ramped-flip-angle approach yields only ~7% signal variation. For these same two excitation schemes, lookup tables to convert signal ratios into T1 values are plotted for a wide range of B1+ scaling factors (0.5-1.5) representing actual flip angle of 10° to 30° (Figure 3). The ramped-flip-angle approach has ~8x reduced sensitivity to errors in B1+ as compared to the constant-flip-angle approach. Correlation and Bland-Altman plots illustrate the relationship between the phantom PDFF-T1 T1 values and spin-echo experiments, showing excellent agreement (Figure 4). Fat fraction and T1 maps from the liver of a healthy control and subject with mild fatty liver disease are compared (Figure 5).DISCUSSION
The proposed PDFF-T1 sequence yielded fat fraction and water T1 maps in a single patient-friendly breath-hold, with significant reduction of unwanted T1-weighting and flip-angle (B1+) dependence with the use of a ramped-flip-angle approach. Minimization of T1-weighing has the advantage of: i) increase fat fraction accuracy and ii) reduced B1+ sensitivity in T1 calculation (Fig. 3). The use of the robust Dixon fat-water separation ensures minimal fat contamination of water-derived T1 values. The PDFF-T1 approach avoids the complex T1 signal response for mixed fat-water systems observed for bSSFP T1 mapping methods. (4,5) The multi-echo acquisition also enables T2* assessment.CONCLUSION
Fast simultaneous quantification of PDFF (with minimal T1 effects) and water T1 is possible with the proposed PDFF-T1 approach. Ramped-flip-angles minimize sensitivity to B1+.Acknowledgements
No acknowledgement found.References
1. Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol 2009;193(1):14-27. 2. Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008;60(5):1122-1134. 3. Chow K, Kellman P, Spottiswoode BS, Nielles-Vallespin S, Arai AE, Salerno M, Thompson RB. Saturation pulse design for quantitative myocardial T1 mapping. J Cardiovasc Magn Reson 2015;17:84. 4. Larmour S, Chow K, Kellman P, Thompson RB. Characterization of T1 bias in skeletal muscle from fat in MOLLI and SASHA pulse sequences: Quantitative fat-fraction imaging with T1 mapping. Magn Reson Med 2017;77(1):237-249. 5. Mozes FE, Tunnicliffe EM, Pavlides M, Robson MD. Influence of fat on liver T1 measurements using modified Look-Locker inversion recovery (MOLLI) methods at 3T. J Magn Reson Imaging 2016;44(1):105-111.Figures
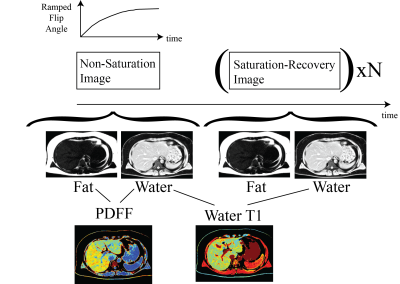
Figure 1: PDFF – T1 Pulse
Sequence and Analysis Approach. Fat
fraction (PDFF) is calculated from a non-saturation acquisition while
water-specific T1 is calculated from saturation and non-saturation recovery
water-separated images. All images incorporated
a ramped flip angle scheme, to minimize sequence-related T1-weighting.
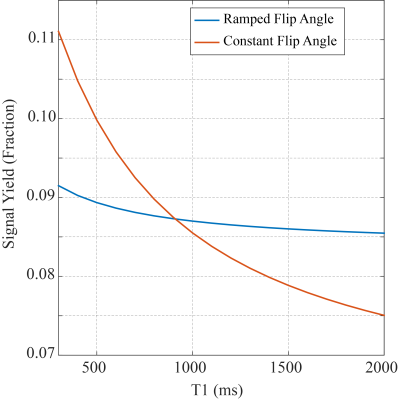
Figure 2: T1-Signal Dependence of a
Spoiled Gradient Echo Sequence (20° Flip) with Constant and Ramped Flip Angles.
Ramped flip angles, following a sin(θ) weighting of the flip angles over the image acquisition, minimize sequence-related T1-weighting in the PDFF-T1 approach. Simulations are for pulse sequence parameters detailed in Methods. These simulations are for the non-saturation image.
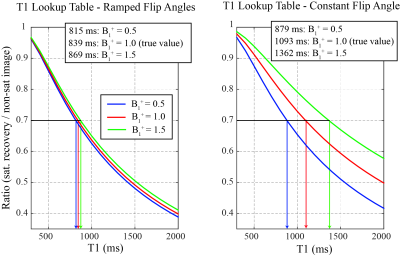
Figure 3: Comparison of T1 Lookup
Table Dependence on B1+
fields for Constant and Ramped Flip Angle Approaches. The use of ramped flip angles, to
minimize sequence-related T1 weighting, significantly reduces the dependence to
lookup-table derived T1 values on the variations in the amplitude of the
transmit radiofrequency field (B1+). The variation in B1+ scaling from 0.5 to 1.5 is equivalent to variations in the prescribed flip angles form 10°-30°, from the targeted 20°.
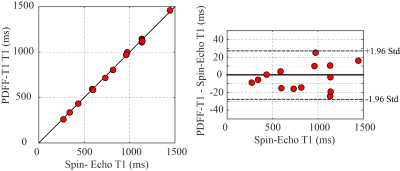
Figure
4: Phantom Studies – Comparison of PDFF-T1 and Gold Standard Spin-Echo T1
experiments. Phantom experiments
illustrate the T1 accuracy of the PDFF-T1 values in phantoms, with comparison
to gold standard spin-echo experiments.
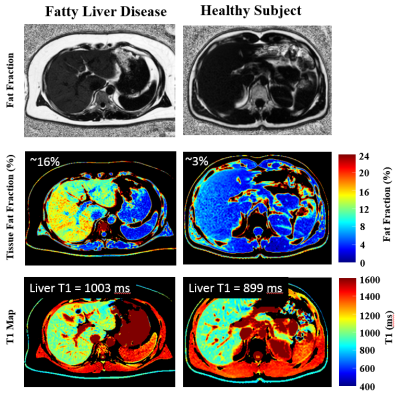
Figure 5: In-Vivo
Studies – PDFF-T1 Studies in a Healthy Control and Subject with Mild Fatty
Liver Disease. The subject with mild fatty liver disease has increase liver
fat fraction (~16%) and increased T1 values as compared to the healthy subject.
Data was acquired in a 13 seconds breath-hold (one of three slices is shown).