0011
A Dual Echo, Dual VENC (DEDV) phase contrast method for Simultaneous Measurement of Myocardial and Blood Flow Velocities1Physics and Texas Center for Superconductivity, University of Houston [Main Campus], HOUSTON, TX, United States, 2Baylor St. Luke's Medical Center, Houston, TX, United States
Synopsis
Dual VENC Velocity quantification has been previously carried out with the two different VENCs acquired in separate repetition cycles (TRs). We propose a magnetic resonance imaging sequence that acquires the phase matrix for two different VENC values in one TR, and use the developed sequence for slow motions and fast flows.
Introduction
Spins moving along the direction of a bi-polar gradient will accumulate a phase shift that is proportional to spin velocity. The velocity sensitivity of such phase velocity mapping (PVM) methods is determined by the first temporal moment of the velocity encoding gradient (M1). For a given M1, the limiting velocity that results in a phase shift of π is called VENC, and spin velocities>VENC appear aliased. As the velocity-to-noise ratio (VNR) is inversely proportional to VENC, there is incentive to keep VENC as low as possible1. In many clinical situations, e.g.,the measured velocities within the field of view can vary by nearly an order of magnitude. As a result, single VENC acquisitions set to encode faster velocities within the field of view (e.g., systolic arterial velocities) have compromised VNR when measuring slower flows (e.g. diastolic or myocardial tissue velocity). Dual VENC implementations rely on acquiring low and high VENC data in separate repetitions cycles (TR), which doubles the acquisition time per velocity encoding direction2.Purpose
In this work, we propose a dual echo acquisition in which the first and second echo are velocity encoded to different VENC values in a single TR, and demonstrate the feasibility of this approach in estimating blood and myocardial tissue velocities for cardiovascular applications.Materials and Methods
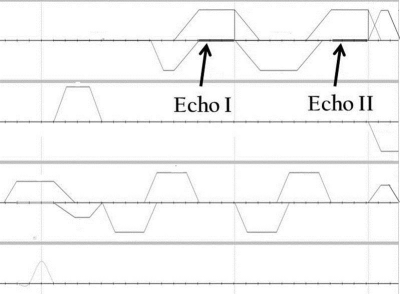
Pulse sequence: A custom pulse sequence capable of applying user prescribed VENC values independently for two echoes was developed in-house (Figure 1), and applied as a patch to the system. The velocity encoded and reference velocity measurements for the dual VENC acquisition were performed in two TRs. The pulse sequence was tested in phantoms and in human volunteers.
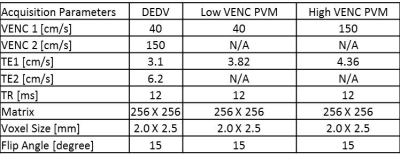
MRI acquisition: All imaging was done at 1.5T and all subjects (n=4, all male, age: 28-40 yrs) provided written informed consent. Following initial scout, a basal short axis slice of the heart was imaged with the following sequences: (i) Conventional PVM with a VENC value of 150 cm/s; (ii) Conventional PVM acquisition with a VENC value of 40 cm/s; and (iii) A DEDV acquisition with VENC values of 150 and 40 cm/s for the first and second echoes respectively. Other acquisition parameters are listed in Figure 5.
Data Analysis: Endo and epi-cardial contours were drawn by an experienced CMR imager on all three PVM acquisitions. Blood and myocardial tissue velocities across cardiac phases was estimated through ROI analysis using custom made software in MATLAB™.
Results
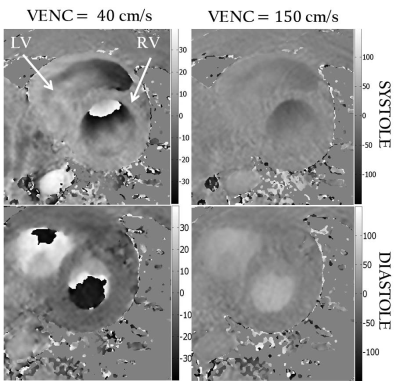
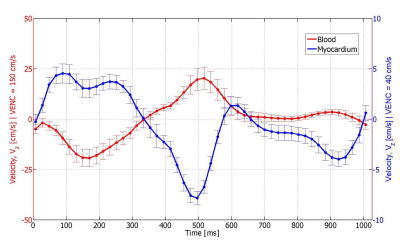
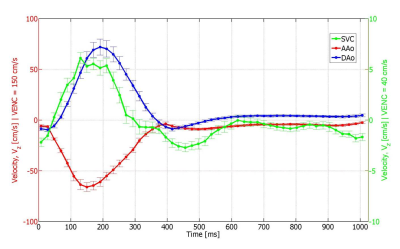
The phase noise in static phantoms conformed to theoretical predictions for the low VENC and high VENC acquisitions as well as in the DEDV acquisition. Compared to the first echo, the second echo in the DEDV sequence had a reduction of 5% in magnitude SNR. Representative images from the DEDV sequence are shown in Figure 2, for through-plane velocity encoding, at the low and high VENC values in diastole and systole. The mean velocity-time curves for the myocardium and the blood in the basal slice are shown in Fig. 3. The positive axis of Vz was defined to point from the basis towards the apex of the heart. The opposed direction of blood flowing through the AAo and DAo confirms information from cardiac anatomy (Figure 4).Discussion
The results from this study show that it is feasible to extract spin velocities that vary by an order of magnitude, e.g., myocardial tissue velocity and blood flow velocity, with sufficient sensitivity within a single TR. Conventional dual VENC approaches encode one VENC value per TR, and the shortest TE/TR combination required for the lower VENC acquisition is longer than that for higher VENC acquisition, resulting in either steady state artifacts (if VENC values are interleaved per TR), or scan inefficiency (using the longer of the two TRs for both VENC acquisitions), or potential for misregistration (if interleaved per cycle, or velocity encoding segments). While the approach described here is for velocity encoding in one direction, the extension to volumetric acquisitions, and multi-directional velocity encoding is straight forward. DEDV acquisition retains all the traditional strengths of dual VENC acquisition, e.g., using the high VENC acquisition to unwrap the aliasing in low VENC acquisition, and improve VNR. It is compatible with under-sampling strategies such as k-t SENSE, or compressed sensing3. A limitation of this DEDV approach is the lack of black blood suppression for the low VENC acquisition, typically used to estimate myocardial velocities.Conclusion
This feasibility study demonstrates the potential of the simultaneous measurement of slow and fast moving tissue velocities using a dual echo dual VENC pulse sequence. This can be used for detailed investigation of the relationship between myocardial motion and flow patterns in different blood chambers.Acknowledgements
No acknowledgement found.References
[1] N. J. Pelc, R. J. Herfkens, A. Shimakawa, and D. R. Enzmann, “Phase contrast cine magnetic resonance imaging,” Magn. Reson. Q., vol. 7, no. 4, pp. 229–254, 1991.
[2] W. B. Buchenberg, M. Markl, S. Bauer, J. Bock, R. Lorenz, and B. A. Jung, “Dual VENC Phase Contrast MRI for Simultaneous Assessment of Blood Flow and Cardiac Motion,” ISMRM, 2011.
[3] J. Tsao, P. Boesiger, and K. P. Pruessmann, “k-t BLAST and k-t SENSE: Dynamic MRI with high frame rate exploiting spatiotemporal correlations,” Magn. Reson. Med., 2003.
Figures