0006
4D-Flow-MRI analysis of aortic flow patterns after replacement of the ascending aorta with a physiologically pre-shaped, 90° bent prosthesis1Department of Radiology and Nuclear Medicine, University Hospital Schleswig-Holstein, Luebeck, Germany, 2Department of Cardiac and Cardiothoracic Vascular Surgery, University Hospital Schleswig-Holstein, Luebeck, Germany, 3Fraunhofer MEVIS, Bremen, Germany
Synopsis
Altered aortic anatomy after prosthesis implantation has been shown to increase secondary flow patterns with potential long-term effects. Therefore, patients after valve-sparing aortic root and ascending aorta replacement with a physiologically pre-shaped prosthesis were examined with 4D-Flow-MRI and compared to patients with straight grafts and age-matched volunteers. A reduced angulation at the distal anastomosis as well as a slightly reduced intensity of secondary flow patterns was confirmed. However, there was no reduction of secondary flow patterns in comparison to straight prostheses potentially attributed to a residual angulation at the proximal anastomosis and a dilatation at the distal anastomosis.
Background and Purpose
The David-procedure is a standard valve-sparing technique for surgical repair of aortic root aneurysms (VSARR). If the aneurysm extends into the ascending aorta, an additional prosthesis of the ascending aorta (AAoR) is implanted. Traditionally, a straight graft is used to replace the physiologically curved ascending aorta. As a result, a kinking at the proximal or distal anastomosis of the AAoR can frequently be observed1. The association between altered aortic arch anatomy and an increased number of secondary flow patterns was shown in previous 4D-Flow-MRI studies2-5. This holds the potential risk of thromboembolisms and unwanted vessel wall remodeling. Physiologically pre-shaped, 90° bent AAoR-prostheses promise to reduce kinking and associated flow alterations. Therefore, it was the purpose of this work to compare flow patterns in patients with VSARR and 90° curved AAoR prostheses (CP; Uni-Graft W Aortic Arch, Braun, Germany) to those with VSARR and straight prostheses (SP) and age-matched, healthy volunteers (VOL).Methods
MRI scans: 28 subjects were included in this HIPPA-compliant study: 9 patients with curved AAoR prosthesis [8m, 62±10y], 8 patients with straight grafts [8m, 60±10y], and 12 healthy volunteers [2m, 55±16y]. Participants were examined at 3 Tesla with a 20-channel body surface coil. A retrospectively ECG-gated, time-resolved, three-dimensional phase contrast-sequence with velocity encoding in all spatial directions (“4D-Flow-MRI”) with adaptive respiratory gating, Venc=180-200cm/s and an isotropic spatial resolution of 2.6mm3, interpolated to 2mm3, SENSE (Reff = 2.1) and Cartesian sampling was acquired. Typical imaging parameters were TR/TE 3.6/2.3ms. Contrast agent (1.0mmol gadobutrol, 0.1ml/kg BW, Bayer HealthCare) was applied in all patients; the flip angle was adapted accordingly. Acquired images were reconstructed to 20 time frames resulting in an effective temporal resolution of 34-61ms depending on each individual’s heart rate (49-87/min). Scan time was 13±3min.
Data analysis: GTFlow (v2.1.15, GyroTools, CH) was used to render the vessel wall and to visualize blood flow using streamlines and time-resolved particle traces, both color-coded with respect to the acquired velocities. Offline data processing included aliasing correction in 5 datasets using PhaseUnwrappingTool (v1, Fraunhofer MEVIS, GER). Aortic geometries known to influence hemodynamics (round, gothic, cubic6,7), presence of kinking, and diameters were assessed. Secondary flow patterns8 (vortices, secondary helices) in the thoracic aorta were recorded and graded as 1: <33%, grade 2: ≥33% and ≤66%, and grade 3: >66% according to each pattern’s extent in relation to the vessel diameter. Due to the sample size, statistical significance was tested applying the Mann-Whitney-U-test.
Results
All studies and flow pattern analyses were successfully accomplished. As seen in Fig. 1, angular forms of the thoracic aorta predominated in patients: 67% (CP) and 75% (SP) presented with a cubic form, 22% (CP) and 25% (SP) revealed a gothic aortic arch. Only one CP-patient had a round aortic arch. In contrast, round forms dominated in volunteers (83%). In CP-patients there was significant reduction of a kinking at the distal anastomosis in comparison to patients with straight grafts: 22% (CP), 75% (SP) (p=0.04), see also Fig. 2. All patients but the aforementioned CP-patient with a round arch showed an angulation between the prostheses of aortic root and ascending aorta. At the distal anastomosis, between straight or pre-shaped graft to the aortic arch, there was a dilatation of 0.5±0.4cm (CP) and 0.6±0.4cm (SP) (n.s.), see also Fig. 3. Patients developed more secondary flow patterns than volunteers (CP: n=3.1±1.5; SP: n=3.4±1.1; VOL: n=1.4±0.8; p=0.002). The most pronounced increase of secondary flow patterns in patients was in the region of the prosthesis and directly distal to it (CP: n=2.2±1.4; SP: n=2.3±1.0; VOL: n=0.3±0.5; p<0.001), Fig. 2. There was no significant difference between prosthesis types (p=0.728). The only CP-patient with a round aortic arch developed no prosthesis-related flow patterns but a small, physiological vortex in the ductus diverticulum. In CP secondary flow patterns in and directly distal to the prosthesis tended to be less pronounced than in SP (median [25%,75%], CP: 2[2,2], SP: 2[2,3]; p=0.088).Discussion and Conclusion
The anatomically pre-shaped 90° prosthesis resulted in a reduced kinking at the distal anastomosis and slightly reduced intensity, yet no decrease in number of secondary flow patterns in comparison to patients with a straight graft. The constant number of secondary flow patterns despite an improved postoperative geometry can be explained by the residual angulation at the proximal anastomosis of the curved prosthesis and a post-prosthetic dilatation. This is underlined by findings in a single patient with a curved prosthesis who revealed both a near-physiological geometry and flow patterns comparable to age-matched volunteers.Acknowledgements
The authors express their gratitude toward Mrs. Martina Schroeder for her skillful assistance and Dr Gerard Crelier for his continuous support.References
1.
Misfeld
M et al., Ann Thorac Surg. 2004: 78(3).
2.
Oechtering
TH et al. 2725. ISMRM.
2015.
3.
Bogren
HG et al., J Thorac Cardiovasc Surg. 1995: 110(3).
4. Bogren
HG et al., J Cardiovasc Magn Reson. 2000: 2(3).
5. Frydrychowicz
A et al., Interact Cardiovasc Thorac Surg. 2006: 5(4).
6. Frydrychowicz
A et al., Eur Radiol. 2012: 22(5).
7. Ou
P et al., J Am Coll Cardiol. 2007: 49(8).
8. Kilner
PJ et al., Circulation. 1993: 88.
Figures
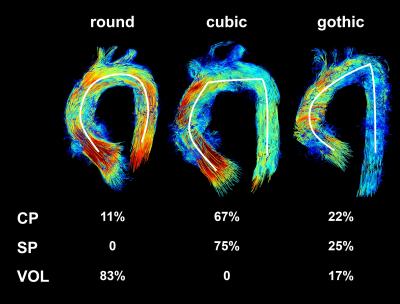
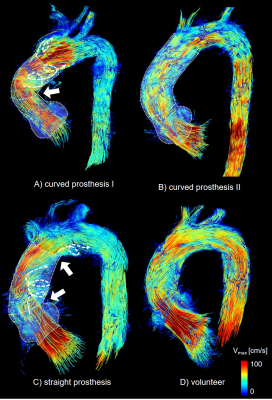
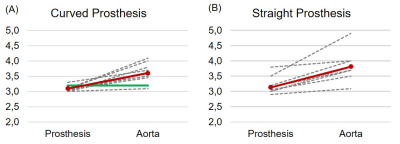
Figure 3 depicts the diameter change between the prosthesis lumen and the native aorta at the distal anastomosis. Red lines depict the mean value of diameter change, the green line indicates the single patient with a curved prosthesis with near-physilogical geomatry (see als Fig. 2B).