Gadolinium in MSK Imaging: Technical Aspects
1Radiology, The Ohio State University College of Medicine, Columbus, OH, United States
Synopsis
This lecture will describe the gadolinium based MRI contrast agents (GBCA) used in musculoskeletal MRI imaging. It will describe basic design and function principles and historical development of the GBCA emphasizing the links between chemical structure and gadolinium dissociation and deposition. Emphasis will also be placed on appreciation of the hierarchy of data from in vivo human to physical chemical numbers like binding constants and kinetics of dissociation. Recent findings and toxicologic studies will be described, as well as a look to the future of the field.
Chemical Principles for GBCA
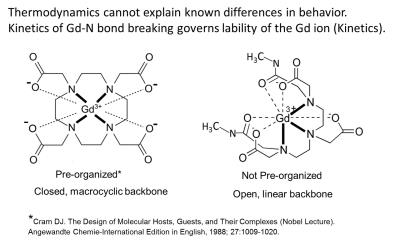
Chemical concepts needed to support the discussion of the Gd based contrast agents (GBCA) are the nature of the bonds between the necessary chelating ligand and the Gd, their number and the shape of the organic framework that holds the fully formed Gd(chelate) together. Two archetypes of structure exist as shown in (Figure 1). (1) Thermodynamic stability constants describe the energy (and entropy) that is involved in making and breaking of all of the bonds in a Gd chelate. The larger the magnitude of Keq or Kf’ (Keq adjusted to physiologic pH), the more tendency that the equilibrium,
Gd3+ + chelate = Gd(chelate) Keq = [Gd(chelate)] / [Gd] [chelate] (pH independent)
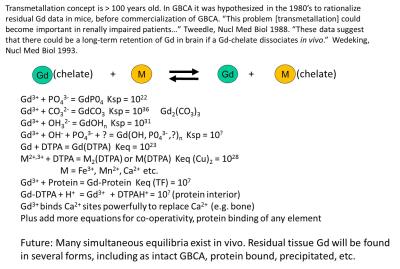
is driven to the right, and all commercial GBCA are driven powerfully rightward. Free Gd ion is toxic in various ways, especially it is insoluble at physiologic pH. In vivo numerous competitors of Gd for the chelating ligand (e.g. endogenous metals), competitors of the chelating ligand for the Gd 3+ ion (e.g. phosphate, hydroxide), and metabolizing enzymes exist, all in equilibrium or attempting to arrive there, and that time of arrival is mitigated by the rapid rate at which the GBCA is being excreted into the urine (90 min halftime).(Figure 2) In addition, though the archetypes differ in their robustness toward resisting some of these biologic attackers. A hierarchy exist in parsing the various data available that is used to describe in vivo behavior and explain it, and this places in human data at the top, and the chemical considerations at the bottom, so while we should know what is feasible in vivo based on chemical principles, we should avoid the trap of extrapolating from bottom to top. GBCA are safer when they are more “stable in vivo,” and not simple when they have larger “stability constants” measured in water.
Historical Perspective
Gd was chosen as the metal for GBCA because it contained the maximum unpaired electrons, 7, while competitor metals, Fe(III) and Mn(II) contained only 5 and would have required higher doses. The Gd(III) also had only one possible oxidation state, while the others could engage in biological redox chemistry that would have been a possible toxicologic pathway. All three bare metals were toxic at the mole doses required for MRI. Dissociated Free Gd did not become an issue except in a few chemists’ minds, who demonstrated between 1988 and 1995 that small amounts of Gd (0.03 – 0.3 % of the injected MRI level dose) remained in mice 1 -2 weeks after dosing, and that endogenous ions could aid dissociation of Gd in vitro, with the macrocycle being more resistant. (2-5) Radiolabeled GBCA did not demonstrate brain 153Gd above LOD, but injected free 153Gd showed brain deposition with very slow clearance, while in the whole mice free Gd3+ did clear at a T1/2 of ~ 50 days. (6) Other data on human skull bone chips were < LOD of 1 ppm. (7) That some Gd was released from gadopentetate in humans from that agent’s earliest formulation was deducible, related to transient, reversible Fe and bilirubin elevations in human blood samples, because adding additional chelating agent to the formulation was the remedy. (8) The formulation of gadodiamide (which showed the same transient elevations in clinical trials) were fortified with 5 mol % of additional chelating agent from the beginning, and this increased the LD50 in mice (9) and reduced the residual Gd remaining in mice after dosing. (3) Prior to NSF connection to Gd in 2006, macrocyclic agents were shown in vitro, in animals and then finally in human bone fragments (10) to lead to lower residual Gd. (But in 2009 Darrah (11) found for the same two agents, no macrocycle vs linear GBCA difference in bone 8 years after exposure). While the weight of the evidence favors dissociated Gd as highly probable, it was not directly detected by 2006, when the link between NSF and Gd was discovered by two groups in the EU. (12, 13) Since then it is now known that the order of decrease from most cases to least cases follows the order of the known residual Gd from the studies above, strongly inferring, though still not proving, that it is dissociated Gd that is one of the triggers. Dissociated Gd was finally proven to form in human serum for linear variants but not macrocycles in 2008. (14) The fact that contraindicating linear GBCA, except for gadobenate that uniquely has an added hepatobiliary path to excretion with otherwise mostly renal excretion, stopped NSF, contributes to the free Gd hypothesis. Dissociated Gd was also found in skin tissue of an NSF patient, where Birka found insoluble Gd, plus gadoteridol, but not gadopentetate, years after dosing the two agents. (15) Gd in biopsy samples is being found paired with Ca and P, suggesting GdPO4 deposition and or replacement of Ca with Gd. With the peak hierarchy fact of NSF added to the medical and toxicologic knowledge base, there was finally serious clinical attention paid to the Gd dissociation phenomenon. Probably this awareness played a role in Kanda’s discovering, in 2014, elevated MRI signal in an area of the brain known to concentrate metal ions, the dentate nucleus. (16)Subsequent studies on ex vivo human autopsy tissue have demonstrated that Gd was indeed present in the brain tissue, the MRI signal correlated to dose, the highest and lowest (or no) signal ordering of GBCA showed that macrocycles have the smallest effect, and also no H&E pathology was obvious. (17-19)
GBCA and Gd in Brain
The brain deposition is now attracting a great deal of attention, (21) (systematic review) and causing changes of specific GBCA use. But is there any evidence to suggest that longer exposure to GBCA and even dissociated Gd at the low levels detected will manifest itself in important toxicity? One thing not clarified in the retrospective human MRI and the human autopsy studies is what fraction of any retained Gd is simply slowly clearing intact GBCA. Two well executed recent rat studies are relevant. One showed that ~50 % of very heavily dosed (12 mmol/kg) gadodiamide cleared the brain over 20 weeks with no H&E neurotoxicity. (22) In a similar but not identical study even more heavily dosed (25 mmol/kg) rat study, ~50% of the same GBCA was found in insoluble and protein bound brain fractions. (23) Protein bound Gd can have a vastly stronger effect on MRI signal that GBCA or insoluble Gd. (1) In the latter study, it was found that three linear agents showed the same speciation, but that only soluble small molecules were detected for the two macrocycles. The mechanisms for brain uptake and clearance are several and complex, and both directions of movement and their kinetics will need to be considered. Another relevant subject will be the findings of Gd in non neural tissue, for example, in up to 23 fold greater amounts in bone compared to brain. (24) The toxicology history for Gd is brief, probably because there is not much to find, and some of it is documented in recent reviews.(25, 26) Very long term exposure to implanted Gd metal might have been associated with rat sarcoma formation (but without significance). (27) To analyze the link between toxicity and free Gd in Gd deposition in brain, we must first find some toxicity there, which will probably be easier to do with dissociated Gd than intact GBCA. Neurotoxicity was not detected until 10 mM gadopentetate in perfused hippocampal brain slices, although but < 1 mM GdCl3 was toxic, but that is still far above what is being detected in brain now. (18, 28) Speciation and toxicology of GBCA and free Gd species are needed using tools like laser ablation ICP-MS, hydrophilic extraction HPLC, and MALDI imaging.(29) Looking to the future, should we re-examine Fe(III) and/or Mn(II) as possibilities for patients that require more than a few examinations, or look at better Gd(III) compounds? This is an expensive question, as these drugs cost over $100 million to develop and most possibilities are generic. We should bear in mind as we search for toxicity of Gd, that GBCA have been extremely useful to patients over the past 30 years, after hundreds of million uses.Acknowledgements
The Stefanie Spielman Foundation and the Wright Center for Innovation in Biomolecular Imaging are gratefully acknowledged for support of this work.References
1. Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99(9):2293-352. PubMed PMID: ISI:000082569800006.
2. Tweedle MF, Gaughan GT, Hagan J, Wedeking PW, Sibley P, Wilson LJ, Lee DW. Considerations Involving Paramagnetic Coordination-Compounds as Useful Nmr Contrast Agents. Nuclear Medicine and Biology. 1988;15(1):31-6. PubMed PMID: ISI:A1988L614900007.
3. Tweedle MF, Wedeking P, Kumar K. Biodistribution of radiolabeled, formulated gadopentetate, gadoteridol, gadoterate, and gadodiamide in mice and rats. Investigative radiology. 1995;30(6):372-80. Epub 1995/06/01. PubMed PMID: 7490190.
4. Tweedle M, Hagan J, Kumar K, Mantha S, Chang C. Reaction of gadolinium chelates with endogenously available ions. Magnetic resonance imaging. 1991;9(3):409-15.
5. Wedeking P, Kumar K, Tweedle MF. Dissociation of Gadolinium Chelates in Mice - Relationship to Chemical Characteristics. Magnetic resonance imaging. 1992;10(4):641-8. PubMed PMID: ISI:A1992JF73400016.
6. Wedeking P, Kumar K, Tweedle MF. Dose-Dependent Biodistribution of [Gd-153]Gd(Acetate)N in Mice. Nuclear Medicine and Biology. 1993;20(5):679-91. doi: Doi 10.1016/0969-8051(93)90039-W. PubMed PMID: ISI:A1993LK30300016.
7. Weinmann H-J, Press WR, Raduchel J, Platzek H, Schmitt-Willich H, Vogler H. Characteristics of Gd-DTPA and new derivatives. In: Bydder G, Felix r, Buchler E, Drayer BP, Niendorf HP, Takahashi M, Wolf K-J, Dinger JC, editors. Contrast Media in MRI The Netherlands: Medicom Europe, B.V.; 1990. p. 19-29.
8. Niendorf HP, Dinger JC, Haustein J, Cornelius I, Alhassan A, Clauss W. Tolerance of Gd-DTPA: clinical experience. In: Bydder GM, Felix R, Bucheler E, Drayer BP, Niendorf HP, Takahashi M, Wolf KJ, editors. Contrast Media in MRI. The Netherlands: Medicom Europe; 1990. p. 31-40.
9. Cacheris WP, Quay SC, Rocklage SM. The relationship between thermodynamics and the toxicity of gadolinium complexes. Magnetic resonance imaging. 1990;8(4):467-81. Epub 1990/01/01. PubMed PMID: 2118207.
10. White GW, Gibby WA, Tweedle MF. Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative-to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investigative radiology. 2006;41(3):272-8. doi: DOI 10.1097/01.rli.0000186569.32408.95. PubMed PMID: ISI:000235632800010.
11. Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ, Ellen Campbell M, Hauschka PV, Hannigan RE. Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics : integrated biometal science. 2009;1(6):479-88. doi: 10.1039/b905145g. PubMed PMID: 21305156. 12. Grobner T. Gadolinium–a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transpl. 2006;21(4):1104-8.
13. Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: Suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. Journal of the American Society of Nephrology. 2006;17(9):2359-62. doi: Doi 10.1681/Asn.2006060601. PubMed PMID: ISI:000240113400005.
14. Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37 degrees C. Investigative radiology. 2008;43(12):817-28. PubMed PMID: ISI:000261271300001.
15. Birka M, Wentker KS, Lusmoller E, Arheilger B, Wehe CA, Sperling M, Stadler R, Karst U. Diagnosis of Nephrogenic Systemic Fibrosis by means of Elemental Bioimaging and Speciation Analysis. Analytical chemistry. 2015;87(6):3321-8. Epub 2015/02/25. doi: 10.1021/ac504488k. PubMed PMID: 25708271.
16. Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014;270(3):834-41. Epub 2014/01/31. doi: 10.1148/radiol.13131669. PubMed PMID: 24475844.
17. Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, Haruyama T, Kitajima K, Furui S. Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy. Radiology. 2015;276(1):228-32. doi: 10.1148/radiol.2015142690. PubMed PMID: 25942417.
18. McDonald RJ, McDonald JS, Kallmes DF, Jentoft ME, Murray DL, Thielen KR, Williamson EE, Eckel LJ. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology. 2015:150025. Epub 2015/03/06. doi: 10.1148/radiol.15150025. PubMed PMID: 25742194.
19. Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and Other Non-Group 1 Gadolinium Contrast Agents Deposit Low Levels of Gadolinium in Brain and Bone Tissue: Preliminary Results From 9 Patients With Normal Renal Function. Investigative radiology. 2016;51(7):447-53. doi: 10.1097/RLI.0000000000000252. PubMed PMID: 26863577.
20. Wedeking P, Shukla R, Kouch Y, Nunn A, Tweedle M. Utilization of the nephrectomized mouse for determining threshold effects of MRI contrast agents. Magnetic resonance imaging. 1999;17(4):569-75.
21. Olchowy C, Cebulski K, Lasecki M, Chaber R, Olchowy A, Kalwak K, Zaleska-Dorobisz U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity - A systematic review. PloS one. 2017;12(2):e0171704. doi: 10.1371/journal.pone.0171704. PubMed PMID: 28187173; PMCID: PMC5302442.
22. Smith APL, Marino M, Roberts J, Crowder JM, Castle J, Lowery L, Morton C, Hibberd MG, Evans PM. Clearance of Gadolinium from the Brain with No Pathologic Effect after Repeated Administration of Gadodiamide in Healthy Rats: An Analytical and Histologic Study. Radiology. 2017;282(3):743-51.
23. Frenzel T, Apte C, Jost G, Schockel L, Lohrke J, Pietsch H. Quantification and Assessment of the Chemical Form of Residual Gadolinium in the Brain After Repeated Administration of Gadolinium-Based Contrast Agents: Comparative Study in Rats. Investigative radiology. 2017. doi: 10.1097/RLI.0000000000000352. PubMed PMID: 28125438.
24. Murata N, Gonzalez-Cuyar LF, Murata K, Fligner C, Dills R, Hippe D, Maravilla KR. Macrocyclic and other non–group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investigative radiology. 2016;51(7):447-53.
25. Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ. Gadolinium-based contrast agents: A comprehensive risk assessment. J Magn Reson Imaging. 2017. doi: 10.1002/jmri.25625. PubMed PMID: 28083913.
26. Semelka RC, Ramalho M, Jay M. Summary of special issue on gadolinium bioeffects and toxicity with a look to the future. Magnetic resonance imaging. 2016;34(10):1399-401. doi: 10.1016/j.mri.2016.09.002. PubMed PMID: 27639920.
27. Haley TJ, Raymond K, Komesu N, Upham HC. Toxicological and pharmacological effects of Gadolinium and samarium chlorides. Brit J Pharmacol. 1961;17:526-32.
28. Taber KH, Bryan RN. The Neurotoxicity of Agents that could be used for contrast-enhanced magnetic resonance imaging. In: Runge VR, Claussen C, Felix R, James AE, editors. Contrast Agents in Magnetic Resonance Imaging. Amsterdam: Excerpta Medica; 1986. p. 70 - 3.
29. Tweedle MF. Gadolinium deposition: Is it chelated or dissociated gadolinium? How can we tell? Magnetic resonance imaging. 2016;34(10):1377-82. doi: 10.1016/j.mri.2016.09.003. PubMed PMID: WOS:000389287000008.
Figures