Investigation & Evaluation of Immunotherapies with Molecular Imaging
1Diagnostic Radiology, Microbiology & Immunology, Physics & Atmospheric Science, School of Biomedical Engineering, Dalhousie University, 2Biomedical Translational Imaging Centre (BIOTIC), Halifax, NS, Canada, 3Physics & Atmospheric Science, Dalhousie University, Halifax, NS, Canada, 4Diagnostic Radiology, Physics & Atmospheric Science, Dalhousie University, Halifax, NS, Canada
Synopsis
Immunotherapies are becoming increasingly important for improved treatment of a variety of cancer types. However, the development of these novel therapies has outstripped our understanding of underlying mechanisms and how best to apply them. It is therefore crucial that we use tools such as MRI, and other molecular imaging techniques, to evaluate immunotherapies in both the clinic and in preclinical studies, and develop new probes and biomarkers to increase their efficacy. This talk will explore the work being done by a number of labs utilizing MRI, including the development of novel probes and biomarkers (both prognostic and diagnostic).
Highlights
Immunotherapies are a broad category of treatments that work by activating the immune system in some fashion to initiate a broad anti-cancer response
A number of immunotherapies have been developed and are in the clinical pipeline, but there is still a lot that is not known about their basic science
MRI and other molecular imaging
techniques can monitor immunotherapies longitudinally to help researchers and
clinicians better understand individual response
- Imaging can be used to monitor therapies, develop new probes to target certain markers, or for novel diagnostic or therapeutic biomarkers
Target Audience
This talk is aimed at scientists and clinicians who are interested in the use of MRI and other molecular imaging techniques (PET/SPECT/optical imaging) for the study and evaluation of immunotherapies. This talk is appropriate for trainees, technical staff, and faculty/MDs.Outcomes/Objectives
At the end of this talk, attendees will be familiar with many of the most commonly used immunotherapies being developed. Attendees will also learn how MRI and other molecular imaging techniques are currently being used to study novel immunotherapies, including the development of novel diagnostic and therapeutic probes and/or biomarkers and monitoring of therapy response.Purpose
To use MRI and other molecular imaging techniques to study immunotherapies, both at the preclinical and clinical level. This can include the study of basic molecular mechanisms, development of novel probes and the study of novel diagnostic and therapeutic biomarkers.Background/Rationale
Therapeutic intervention for cancer no longer adheres to the traditional trio of surgery, chemotherapy and radiation therapy. It is a complex challenge that can be influenced by cancer type and stage, and the host immune response. A broad range of new therapies are being developed due to a large number of patients who do not respond, or only have partial responses, to traditional therapies such as chemotherapy and radiation.
Newer therapeutic classes include: personalized medicines that are targeted to the biologic or genetic makeup of the cancer strain or patient; targeted therapies that are designed to interfere with specific molecular targets in cancer cell growth or progression; therapeutic cancer vaccines that are designed to harness the immune system to fight cancer; and preventative vaccines which target viruses or other infectious agents that lead to cancer development. Many targeted therapies, therapeutic and preventative vaccines can be classified as immunotherapies.
Immunotherapies are novel in that they don’t necessarily target the tumor directly, but instead use the immune system to target tumors(1). Immunotherapies comprise one of the most important and fastest growing classes of cancer therapies, with Science magazine naming cancer immunotherapy “the breakthrough of the year” in 2013(2). From monoclonal antibodies to peptide-based vaccines, the importance of immunotherapy cannot be understated. It is no longer a niche field applying only to a subset of cancers, but rather has the potential to become an equal pillar to chemotherapy and radiation therapy as frontline treatments for cancer(1). Immunotherapy is also being increasingly combined with more traditional chemotherapy to maximize effects (3-9). These so-called combination therapies are often synergistic, offering increased responses over either therapy used in isolation.
The study of immunotherapy in cancer has had several clinical successes over the last few years, with several monoclonal antibodies, cytokines, checkpoint inhibitors and a peptide-based vaccine being approved for clinical use(10-15). As preclinical research leads into clinical trials, exploring the potentially unique mechanism of action of these new immunotherapy candidates is critical to the interpretation of clinical responses and will aid in clinical trial design, particularly given that the mechanism of immunotherapy is unique from that of chemotherapy.
One of the disadvantages of the rate of clinical immunotherapy studies is that it has outpaced our understanding of the underlying basic science(11). Therefore it is crucial that we continue to study the underlying mechanisms to improve the development of novel therapies as well as the implementation of current therapies. Studies have shown high degrees of variability in individual response to cancer, increasing the necessity of a more personalized approach(11).
Methods
This talk will touch on a number of molecular imaging methods used to study immunotherapy response(16-22). We will discuss the use of MRI cell tracking for monitoring both adoptive cell immunotherapies, and immune cell population migration in response to other immunotherapy subtypes (such as anti-PD1 checkpoint inhibitor and a peptide-based vaccine). We will also discuss the use of PET (using standard FDG imaging, and novel probes specifically developed for immunotherapies) and PET/MRI multimodal imaging for monitoring both anatomical and functional changes with MRI (using DCE, T1/T2-weighted imaging, etc.)Results
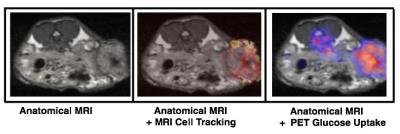
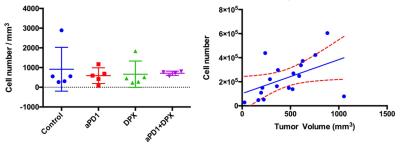
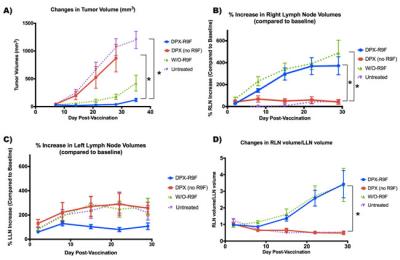
This talk will discuss results that have been generated by a number of groups studying immunotherapies including results generated in my lab. Some of the results to be discussed from my group include the use of PET/MRI to monitor necrotic regions with PET, and MRI cell tracking of labeled immune cells (Figure 1). We have also studied how these populations of immune cells change in response to treatment with different immunotherapies (Figure 2). Other work demonstrates that peptide-based vaccines can cause localized volumetric changes to lymph nodes (23), which can be a predictor of therapy response and a potential therapeutic biomarker (Figure 3).Discussion
Aside from demonstrating the wide range of work already being done to study immunotherapies, I will also touch on some of the barriers that imaging has to overcome in order to achieve wider uptake, including problems such as differential tumor response to immunotherapies vs chemo/radiation therapy which has necessitated changes to classical clinical guidelines such as RECIST (such as the new iRECIST (24)).Conclusion
Immunotherapies are becoming increasingly important for improved treatment of a variety of cancer types. However, the development of these novel therapies has outstripped our understanding of underlying mechanisms and how best to apply them. It is therefore crucial that we use tools such as MRI, and other molecular imaging techniques, to evaluate immunotherapies in both the clinic and in preclinical studies, and develop new probes and biomarkers to increase their efficacy.Acknowledgements
We'd like to acknowledge technical assistance and slides from Christa Davis, Andrea West, Alecia McKay, Genevieve Weir and Marianne Stanford.References
1. Gravitz L. Cancer immunotherapy. Nature 2013;504:S1–S1. doi: 10.1038/504s1a.
2. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432.
3. Andersen MH, Junker N, Ellebaek E, Svane IM, Thor Straten P. Therapeutic cancer vaccines in combination with conventional therapy. J. Biomed. Biotechnol. 2010;2010:237623–10. doi: 10.1155/2010/237623.
4. Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother 2009;58:317–324. doi: 10.1007/s00262-008-0576-4.
5. Benz MR, Czernin J, Allen-Auerbach MS, et al. FDG-PET/CT Imaging Predicts Histopathologic Treatment Responses after the Initial Cycle of Neoadjuvant Chemotherapy in High-Grade Soft-Tissue Sarcomas. Clinical Cancer Research 2009;15:2856–2863. doi: 10.1158/1078-0432.CCR-08-2537.
6. Chen C-A, Ho C-M, Chang M-C, Sun W-Z, Chen Y-L, Chiang Y-C, Syu M-H, Hsieh C-Y, Cheng W-F. Metronomic chemotherapy enhances antitumor effects of cancer vaccine by depleting regulatory T lymphocytes and inhibiting tumor angiogenesis. Mol. Ther. 2010;18:1233–1243. doi: 10.1038/mt.2010.34.
7. Engles JM, Quarless SA, Mambo E, Ishimori T, Cho SY, Wahl RL. Stunning and its effect on 3H-FDG uptake and key gene expression in breast cancer cells undergoing chemotherapy. J Nucl Med 2006;47:603–608.
8. Pfannenstiel LW, Lam SSK, Emens LA, Jaffee EM, Armstrong TD. Paclitaxel enhances early dendritic cell maturation and function through TLR4 signaling in mice. Cellular Immunology 2010;263:79–87. doi: 10.1016/j.cellimm.2010.03.001.
9. Weir GM, Hrytsenko O, Stanford MM, Berinstein NL, Karkada M, Liwski RS, Mansour M. Metronomic cyclophosphamide enhances HPV16E7 peptide vaccine induced antigen-specific and cytotoxic T-cell mediated antitumor immune response. Oncoimmunology 2014;3:e953407.
10. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–489. doi: 10.1038/nature10673.
11. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017;541:321–330. doi: 10.1038/nature21349.
12. Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012;62:309–335. doi: 10.3322/caac.20132.
13. Li X, Hu W, Zheng X, et al. Emerging immune checkpoints for cancer therapy. Acta Oncol 2015;54:1706–1713. doi: 10.3109/0284186X.2015.1071918.
14. Kakarla S, Gottschalk S. CAR T cells for solid tumors: armed and ready to go? Cancer J 2014;20:151–155. doi: 10.1097/PPO.0000000000000032.
15. Scott AM, Allison JP, Wolchok JD. Monoclonal antibodies in cancer therapy. Cancer Immun 2012;12:14–511. doi: 10.1146/annurev.iy.07.040189.002405.
16. Gaudet JM, Hamilton AM, Chen Y, Fox MS, Foster PJ. Application of dual (19) F and iron cellular MRI agents to track the infiltration of immune cells to the site of a rejected stem cell transplant. Magn Reson Med 2016. doi: 10.1002/mrm.26400.
17. Makela AV, Murrell DH, Parkins KM, Kara J, Gaudet JM, Foster PJ. Cellular Imaging With MRI. Top Magn Reson Imaging 2016;25:177–186. doi: 10.1097/RMR.0000000000000101.
18. Zhang X, de Chickera SN, Willert C, et al. Cellular magnetic resonance imaging of monocyte-derived dendritic cell migration from healthy donors and cancer patients as assessed in a scid mouse model. Cytotherapy 2011;13:1234–1248. doi: 10.3109/14653249.2011.605349.
19. Marcus CD, Ladam-Marcus V, Cucu C, Bouche O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol 2009;72:217–238. doi: 10.1016/j.critrevonc.2008.07.012.
20. James ML, Gambhir SS. A Molecular Imaging Primer: Modalities, Imaging Agents, and Applications. Physiological Reviews 2012;92:897–965. doi: 10.1152/physrev.00049.2010.
21. LiAnning, WuYue, TangFeng, LiWei, FengXiaoyuan, YaoZhenwei. In Vivo Magnetic Resonance Imaging of CD8+ T Lymphocytes Recruiting to Glioblastoma in Mice. Cancer Biother Radiopharm 2016;31:317–323. doi: 10.1089/cbr.2016.2061.
22. Aarntzen EHJG, Srinivas M, De Wilt JHW, et al. Early identification of antigen-specific immune responses in vivo by [18F]-labeled 3“-fluoro-3-”deoxy-thymidine ([18F]FLT) PET imaging. Proc Natl Acad Sci U S A 2011;108:18396–18399. doi: 10.1073/pnas.1113045108.
23. Brewer KD, DeBay DR, Dude I, et al. Using lymph node swelling as a potential biomarker for successful vaccination. Oncotarget 2016;7:35655–35669. doi: 10.18632/oncotarget.9580. 24. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. The Lancet Oncology 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8.
24. Seymour L, Bogaerts J, Perrone A, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. The Lancet Oncology 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8.
Figures