Introduction to CEST & Quantification
1Harvard University
Synopsis
CEST MRI has emerged as a sensitive contrast mechanism for several metabolites such as glucose, glycogen, creatine and glutamate, as well as tissue pH. It has promising applications in a host of disorders including acute stroke, epilepsy and tumor. As we make the transition from CEST-weighted MRI toward quantitative in vivo CEST imaging for improved characterization of the underlying physiology, it is helpful to review persistent progress in the field of CEST imaging from equations, cells, rodents and patients.
Purpose
To summarize CEST optimization and quantification techniques, aiding ongoing in vivo CEST imaging.Introduction
CEST MRI is a versatile yet relatively complex technique. The measured CEST-weighted image is under the influence of not only CEST properties, but also relaxation time and scan parameters. Briefly, in addition to concentration of compounds and exchange rate, often variables of the interest, CEST effect varies with field strength, saturation waveform, saturation power, saturation duration as well as repetition time and flip angle. It is worthwhile to point out that in vivo CEST MRI often also involves changes in multiple parameters of interest (e.g., protein level, pH and relaxation), making the reverse problem of solving the underlying system somewhat challenging. Nevertheless, persistent progress has been achieved over the years.Quantitative CEST (qCEST)
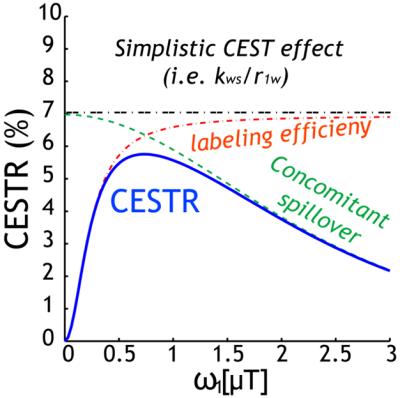
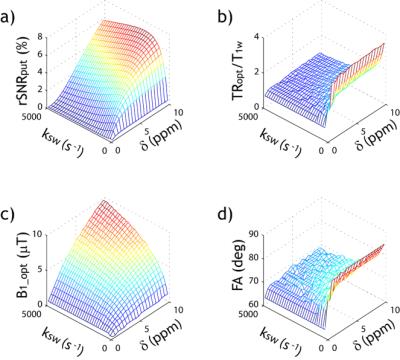
The classical 2-pool CEST effect can be generally described by CESTR=fs*ksw*alpha*(1-sigma)/R1w, where ksw and fs are labile proton exchange rate and concentration relative to bulk water, respectively (Fig. 1). This confers CEST imaging sensitivity to compound concentration and pH/temperature. CEST effect also depends on relaxation, water content, and experimental conditions via the experimental factors. Specifically, alpha is saturation efficiency, which depends on B1 and exchange rate while sigma is the spillover effect that most prominently varies with the chemical shift in Hz (ppm*field), B1, and relaxation. In addition, the saturation transfer from labile proton to bulk water signal approaches the steady state approximately following the classical exponential function. Recently, the full solution of 2-pool CEST effect also includes factors such as TR and flip angle, permitting direct calculation of SNR per unit time for optimizing CEST scan (Fig. 2)1. Worth mentioning are analytical techniques such as QUEST, QUESP, QUESTRA, ratiometric analysis, omega plot, spill-over corrected omega plot and inverse Z-spectrum analysis. Notably, the adoption of T1rho theorem to CEST MRI simplifies quantitative description of multi-pool CEST effect, critical for in vivo quantification. The multi-pool analysis allows resolving contributions from amides (proteins and peptides), amines (creatine, glutamate), NOE (proteins, structure), and semi-solid MT.Exogenous CEST Imaging
Dynamic CEST imaging captures CEST signal change following administration of CEST agents to probe certain tissue characteristics. Most notably, glucose has a unique CEST signature, which is associated with tissue metabolism and cell division cycle. Indeed, glucose CEST (GlucoCEST) imaging has been demonstrated in resolving heterogeneous glucose consumption in tumor, even in cases without Gd enhancement. Another promising application of diaCEST agent is ratiometric CEST imaging with common CT contrast agents such as iopamidol, which provides concentration-independent pH imaging in tumor and kidney2. In addition, there is ongoing efforts to develop exogenous CEST imaging to measure important biological indices, such as pH, temperature, lactate or glucose concentration, enzyme activity or design agents suitable for cell-labeling. For example, reporter gene CEST MRI has been demonstrated to monitor oncolytic viral activity using lysine rich protein (LRP) production3.Endogenous CEST Imaging
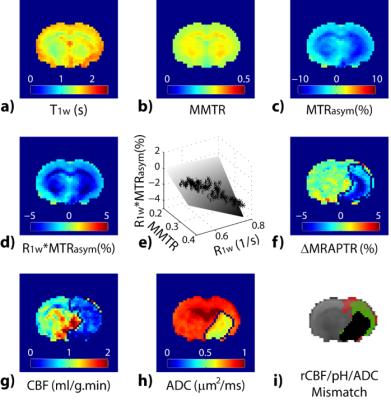
Endogenous CEST relies on natural metabolites/proteins/peptides for tissue characterization. There is a growing interest of the endogenous CEST applications for mapping metabolites or microenvironment properties such as glycogen in the liver, glycosaminoglycans in cartilage, myo-inositol in brain and amide proton transfer (APT) for pH and ensemble protein level. APT-weighted imaging captures composite changes in amide proton content, NOE and relaxation changes. Whereas it has been recognized that MTRasym includes multiple sources of contribution, it has played important role in shaping the field of CEST MRI. For example, it has been shown that the APT-weighted MRI can be used for tumor grading and resolving recurrent tumor from necrosis and edema following treatment, augmenting routine MRI. To resolve the complex contrast in APT-weighted MRI, analytical methods that correct for spillover and semi-solid MT effects or decouples individual CEST effects have been proposed and demonstrated. The results revealed that in addition to APT, NOE4 and Cr5 may serve as imaging biomarkers in tumor. Another promising application of APT MRI is acute stroke. The pH-sensitive APT MRI demarcates graded metabolic disruption in acute ischemic lesion, complementing routine perfusion and diffusion MRI. In addition to APT MRI at 3.5 ppm, amine and amide concentration-independent detection (AACID) tissue pH imaging has been proposed.6 The recently developed magnetization transfer and relaxation-normalized APT (MRPAT) takes the advantage of multi-parameter regression to enhance tissue pH specificity, permitting automated lesion segmentation and improved quantification of tissue acidification (Fig. 3)7. A recent study using multi-pool Lorentzian fitting reported NOE signal in rat brain as a new potential contrast for assessment of acute stroke.8 New means of APT imaging are emerging in studies of multiple sclerosis, breast cancer, prostate and bladder cancer and spinal cord imaging. Amine protons from free amino acids or protein and peptide side chains are another important class of endogenous CEST MRI. Glutamate (Glu) is a major excitatory neurotransmitter in the brain and shows a pH and concentration-dependent CEST effect (GluCEST) between its amine group and bulk water. GluCEST detected glutamate decrease in ischemic stroke9, tumor9, Alzheimer’s Disease (AD)10, and Huntington's disease11. Notably, Glutamate is elevated in epileptic foci. GluCEST sensitively detects lateralized glutamate changes in patients with non-lesional temporal lobe epilepsy (TLE), showing promises for guiding diagnosis and treatment12. Cardiac dysfunction is associated with myocardial ATP loss. ATP is derived from the conversion of phosphocreatine to creatine catalyzed by creatine kinase. CrCEST has been used to map creatine distribution in the myocardium to assess metabolic activity in normal and infarcted animal hearts13,14.Acknowledgements
No acknowledgement found.References
[1] Jiang W, et al. Contrast media & molecular imaging 2016;11:415-23.
[2] Longo DL, et al. Journal of the American Chemical Society 2014;136:14333-6.
[3] Farrar CT, et al. Radiology 2015;275:746-54.
[4] Heo HY, et al. J Magn Reson Imaging 2016;44:41-50.
[5] Cai K, et al. NMR in biomedicine 2015;28:1-8.
[6] McVicar N, et al. Journal of cerebral blood flow and metabolism 2014;34:690-8.
[7] Guo Y, et al. NeuroImage 2016;141:242-9.
[8] Zhang XY, et al. Magnetic resonance imaging 2016;34:1100-6.
[9] Cai K, et al. Nature medicine 2012;18:302-6.
[10] Haris M, et al. NMR in biomedicine 2013;26:386-91.
[11] Pepin J, et al. NeuroImage 2016;139:53-64.
[12] Davis KA, et al. Science translational medicine 2015;7:309ra161.
[13] Zhou Z, et al. Journal of Cardiovascular Magnetic Resonance 2016;18:O70.
[14] Haris M, et al. Nature medicine 2014;20:209-14.
Figures