Cerebrovascular Reserve Imaging
Synopsis
To understand the hemodynamic changes due to a decrease in cerebral perfusion pressure (CPP), and evaluation of cerebrovascular reserve (CVR) capacity in patients with cerebrovascular disease (CVD) is important to determine the risk of future ischemic events and in the selection and planning of the therapeutic interventions. 3 approaches (positron emission tomography, single photon emission computed tomography and MRI) can be used in the evaluation of CVR. We will present the basic concept to measure CVR in patients with CVD combined by nuclear medicine imaging and introduce the possibility of MRI application in measuring CVR.
Purpose
- To understand the mechanism of the hemodynamic cerebral ischemia due to a decrease in cerebral perfusion pressure.
- To review the basical approach to measure cerebrovascular reserve (CVR) applied by nuclear medicine imaging in patients with cerebrovascular disease.
- To study of CVR capacity by magnetic resonance perfusion weighted imaging.
Methods
It is essential to understand the hemodynamic changes due to a decrease in cerebral perfusion pressure (CPP), and evaluation of these compensatory mechanisms is important in patients with cerebrovascular disease (CVD) to determine the risk of future ischemic events and in the selection and planning of the therapeutic interventions.
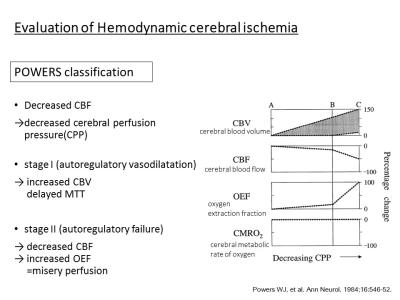
Powers et al. proposed a 2-stage classification of hemodynamic impairment in patients with CVD. As CPP decrease, cerebral blood volume (CBV) increase to maintain the blood flow (stage I; autoregulatory vasodilatation). The capacity of increasing CBV means cerebrovascular reserve (CVR). Increases of CBV and mean transit time (MTT) are 2 parameters that reflect this initial phase of compensatory autoregulatory vasodilatation. Further decreased of CPP beyond cerebral autoregulatory vasodilatation capacity eventually result in stage II (autoregulatory failure), characterized by decreases of CBF and increases of oxygen extraction fraction (OEF), which has been termed “misery perfusion”1).
Generally, 3 approaches (positron emission tomography (PET), single photon emission computed tomography (SPECT) and MRI) can be used in the evaluation of CVR in patients with CVD. The current "gold standard" for the evaluation of cerebral perfusion in humans remains PET study, which can quantitatively measure cerebral blood flow (CBF), CBV, and oxygen consumption. However, arterial sampling is required to measure the arterial tracer concentration and the limited availability of PET centers with a cyclotron, which is limitation of its use for clinical purposes.
The second approach attempts to determine the degree of CVR by comparing CBF under baseline conditions and after a vasodilatory stimulus such as Acetazolamide (ACZ). SPECT study by applying both baseline and ACZ loading can be commonly used to estimate CVR, which is calculated as the percentage increase in CBF after ACZ relative to baseline.
$$ CVR = \frac{CBF(Post-ACZ)-CBF (baseline)}{CBF (baseline)} \times100$$
Vascular territories in affected side undergo compensatory vasodilatation up to a maximal level, which precludes further dilatation of the arteries in the affected region in response to ACZ. Therefore, the expected normal increases of CBF by ACZ loading are blunted compared with normal brain parenchyma.
On the basis of studies using SPECT, MRI perfusion imaging with ACZ loading has been also applied. MRI can detect the changes in magnetic susceptibility during passage of a compact bolus injection of contrast and can yield relative and absolute hemodynamic values of brain perfusion. And also, the measurement of the CBV/CBF ratio, mathematically equivalent to the MTT can be applied by MRI. The increased MTT has reported to correlate with the decreased or no relative CBF increase after ACZ stimulation2).
During this presentation, we will introduce the underlying concept to measure CVR in patients with CVD combined by nuclear medicine imaging and propose the possibility of MRI application in measuring CVR.
Acknowledgements
No acknowledgement found.References
- Powers WJ, Press GA, Grubb RL Jr, Gado M, Raichle ME. The effect of hemodynamically significant carotid artery disease on the hemodynamic status of the cerebral circulation. Ann Intern Med. 1987; 106:27-34.
- Gibbs JM, Wise RJ, Leenders KL, Jones T. Evaluation of cerebral perfusion reserve in patients with carotid-artery occlusion. Lancet. 1984; 1:310-4.