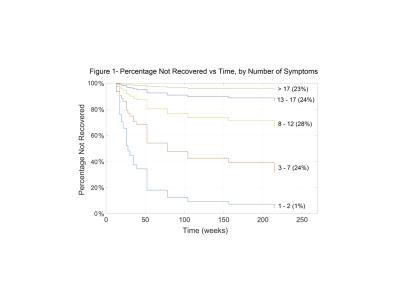
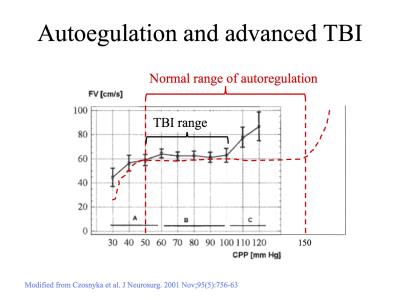
Synopsis
Blood flow dysregulation is known to occur immediately after traumatic
brain injury. Since neurovascular coupling is an essential component for maintaining
the health of the neurovascular unit, impairment of this important regulatory mechanism
can have significant implications on recovery from injury and may therefore be
involved in the persistence of symptoms after injury. The ability to map dysregulation
of blood flow using BOLD MRI cerebrovascular reactivity mapping offers the
ability to investigate blood flow control providing a method to further
understanding the relationship between post-injury blood flow derangements and
recovery from injury.
Acknowledgements
No acknowledgement found.References
1. Hiploylee C,
Dufort PA, Davis HS, Wennberg RA, Tartaglia MC, Mikulis D,
Hazrati LN, Tator CH. Longitudinal Study of Postconcussion
Syndrome: Not Everyone
Recovers. J Neurotrauma. 2016 Nov 29. [Epub ahead of print]
PubMed PMID:
27784191.
2. Blennow K, Hardy J, Zetterberg H. The
neuropathology and neurobiology of traumatic brain injury. Neuron. 2012 Dec
6;76(5):886-99. doi:10.1016/j.neuron.2012.11.021. Review. PubMed PMID:
23217738.
3. Toth P,
Szarka N, Farkas E, Ezer E, Czeiter E, Amrein K, Ungvari Z, Hartings
JA,
Buki A, Koller A. Traumatic brain injury-induced autoregulatory dysfunction
and
spreading depression-related neurovascular uncoupling: Pathomechanisms,
perspectives,
and therapeutic implications. Am J Physiol Heart Circ Physiol. 2016
Nov
1;311(5):H1118-H1131. doi: 10.1152/ajpheart.00267.2016. Review. PubMed PMID:
27614225.
4. Czosnyka M,
Smielewski P, Piechnik S, Steiner LA, Pickard JD. Cerebral
autoregulation
following head injury. J Neurosurg. 2001 Nov;95(5):756-63. PubMed
PMID:
11702864.
5 Vavilala, M., Lee, L., Boddu, K., Visco, E.,
Newell, D., Zimmerman, J., and Lam, A. (2004). Cerebral autoregulation in
pediatric traumatic brain injury. Pediatr. Crit. Care Med. 5, 257–263.
6. Bartnik-Olson
BL, Holshouser B, Wang H, Grube M, Tong K, Wong V, Ashwal S.
Impaired
neurovascular unit function contributes to persistent symptoms after
concussion:
a pilot study. J Neurotrauma. 2014 Sep 1;31(17):1497-506. doi:
10.1089/neu.2013.3213.
PubMed PMID: 24735414.
7. Tang, L., Ge, Y., Sodickson, D. K., Miles,
L., Zhou, Y., Reaume, J., and Grossman, R. I. (2011). Thalamic resting-state
functional net- works: disruption in patients with mild traumatic brain injury.
Radi- ology 260, 831–840.
8 Enevoldsen EM, Jensen FT: Autoregulation and
CO2 responses of cerebral blood flow in patients with acute severe head injury.
J Neurosurg 48:689–703, 1978.
9. Meixensberger J: Xenon 133—CBF measurements
in severe head injury and subarachnoid haemorrhage. Acta Neurochir Suppl 59:28–33, 1993.
10. Steiger HJ, Aaslid R, Stooss R, et al:
Transcranial Doppler monitoring in head injury: relations between type of injury,
flow velocities, vasoreactivity, and outcome. Neurosurgery 34:79–86, 1994.