MR Enterography
1Department of Diagnostic Imaging, The Hospital for Sick Children, Toronto, ON, Canada, 2Department of Medical Imaging, University of Toronto, Toronto, ON, Canada
Synopsis
Magnetic resonance (MR) enterography is a robust alternative to modalities utilizing ionizing radiation in evaluating small bowel and surrounding structures in children and adults. Technical advances enabling rapid relatively motion-insensitive MR sequences and protocol modifications adapted to patients’ ages and morbidities will be detailed. Image optimization improves diagnostic capabilities, further enhanced by systematic review. Image interpretation, from detection and characterization to quantification of disease burden in inflammatory bowel disease (IBD) - and increasingly other bowel disorders - will be discussed. The expanding role MR enterographic findings play as imaging biomarkers in the management of IBD will also be considered.
Learning objectives
1. Tailor MR enterography protocol & technique to patient age and morbidity
2. Apply a systematic approach to image review and reporting
3. Understand role of MR enterographic findings as imaging biomarkers in guiding treatment in inflammatory bowel disease
Introduction
Magnetic Resonance Enterography (MR Enterography) is an abdominal MRI optimized for the small intestine. This is achieved through use of oral contrast agents for lumen distension, cinematic sequences to assess motility with antiperistaltic agents to reduce motion artifact, and use of anatomic and fluid sensitive sequences, together with gadolinium-based contrast agents, to evaluate the bowel wall and surrounding structures.[1]
Over the past decade use of MR Enterography has increased, particularly in children, and is now advocated as the primary imaging modality in the diagnosis and management of patients with inflammatory bowel disease (IBD).[2-4] This growth has resulted from a combination of factors including worldwide increase in the incidence of IBD, technical improvements reducing artifacts and acquisition times while retaining contrast and spatial resolution across field strengths, concerns regarding ionizing radiation in younger patients, and increased utilization of MR enterography findings as imaging biomarkers to guide therapy.[5-7] However, accessibility, cost and procedure time relative to alternative imaging techniques such as ultrasound and computed tomography remain issues. Current imaging pathways typically integrate MR enterography with other modalities, the choice varying with indication and timing during the course of patient care.[3]
While IBD remains the most common indication for MR Enterography to assess small bowel and help distinguish Crohn’s disease from ulcerative colitis, additional information can be obtained about colonic and perianal disease. [8,9] As well, there is expanding interest in MR enterography in other intestinal pathologies including polyposis syndromes and enteropathies e.g. scleroderma and celiac disease.[10]
Standard and Advanced Techniques
Patient education aids image optimization through practicing breath-holding and encouraging adequate fasting of 4-6 hours, reducing artifact from motion and gas. Oral contrast agents, usually biphasic solutions, provide increased lumen signal on T2 weighted sequences and decreased signal on T1 weighted sequences, administered over one hour pre-MRI in 3 divided doses totaling 1300-1500 ml, less in patients under 50 kg where the volume is weighted-based. Antispasmodic agents can be administered in 1 or 2 doses, usually as intravenous boluses although occasionally slow infusion or by intramuscular injection, with doses also weight-based, as are intravenous gadolinium-based contrast agents (GBCA).[11]
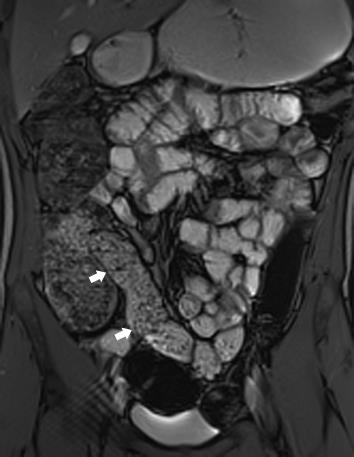
Standard sequences and imaging planes are summarized in Table 1, and can be performed on 1.5 and 3 Tesla scanners. Simple measures to improve image quality include having the patient void pre scan and allowing adequate time for optimum filling of the terminal ileum prior to antispasmodic injection. (Figures 2 and 3)
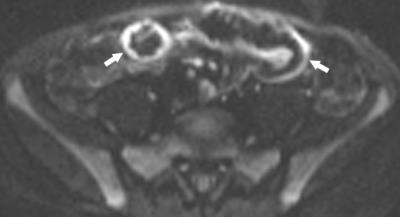
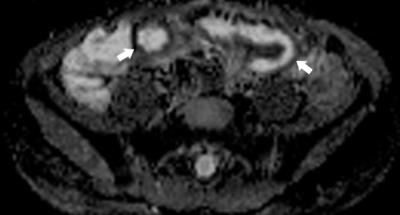
Post contrast imaging is acquired at multiple time points; standardly 45 seconds in the enteric phase, 70 seconds in the portal venous phase, and increasingly delayed imaging to 7 minutes. This offers the potential to distinguish inflammation and fibrosis through enhancement gain.[12] Diffusion weighted imaging (DWI) is now also standardly acquired with increasing utility in depicting active bowel inflammation (Figures 4 and 5), as well as abscesses, best when interpreted in conjunction with conventional sequences.[8, 13, 14] Number and b-value strengths vary. Magnetization transfer (MT) ratios are calculated from imaging acquired with and without an MT pulse, used to indirectly measure mural fibrosis. Although not yet standard, it shows promise.[1,8,11]
Image Analysis
Systematic analysis of MR enterography requires careful comparison of the bowel in different planes and at different points to ensure positive findings are persistent. Diagnosis, characterization and identifying complications in IBD are key goals of imaging in addition to assessing treatment response.
Jejunum evaluation can be aided by review of initial surveillance and cinematic sequences when often better distended than later in the scan. Close correlation of pre and post contrast 3D T1 weighted gradient echo sequences can reveal bright signal from stool mimicking enhancement, fold frequency - which can be reversed in celiac disease in adults, and patterns of wall enhancement, and grade of mural and mesenteric enhancement may be better appreciated.[10]
A standardized checklist and report template for MR enterography can promote consistency in initial reporting and follow-up, particularly relevant in IBD. Quantitative analysis is increasingly used to define disease burden and treatment response, not limited to segment length, leading to the development of MR enterography-based scoring systems in adults and children.[7,8]
Image Interpretation - Imaging Biomarkers
In IBD, mural features closely related to active inflammation include bowel wall thickening ≥ 3mm, T2 hyperintensity, mural and/or striated enhancement, restricted diffusion and normal or reduced peristalsis. Extramural features in Crohn’s disease include fibrofatty proliferation, engorgement of the vasa recta and mesenteric edema and enhancement. Damage can be manifest by fibrostenotic disease with persistent luminal narrowing +/- prestenotic dilatation, often with low T2 mural signal and transmural enhancement but less restriction; and fistulae and abscesses, occurring less often in children.
Experience gained from imaging bowel in IBD is now being applied more widely to improve recognition and monitoring of a range of other bowel pathologies.
MR enterographic findings in IBD are increasingly employed to guide therapy. Active inflammation may warrant anti-inflammatory therapy; fibrostenotic disease dilation or resection; penetrating disease antibiotics and anti-TNF (tissue necrosis factor) therapy or immune modulators; and abscesses antibiotics &/or drainage.[7, 8]
Future Developments
Moving forward, it is likely MR enterography protocols will be refined to better target the clinical question. This may limit comprehensive evaluation to initial review, abbreviated protocols to follow-up - already happening in some centers, and use of different sequences such as MT if surgical intervention is planned, likely with less reliance on GBCA and greater reliance on DWI.Acknowledgements
No acknowledgement found.References
1. Grand DJ, Guglielmo FF, Al-Hawary MM (2015) MR enterography in Crohn's disease: current consensus on optimal imaging technique and future advances from the SAR Crohn's disease-focused panel Abdom Imaging 40(5):953-64.
2. de Bie CI, Buderus S, Sandhu BK et al and EUROKIDS Porto IBD Working Group of ESPGHAN (2012) Diagnostic workup of paediatric patients with inflammatory bowel disease in Europe: results of a 5-year audit of the EUROKIDS registry J Pediatr Gastroenterol Nutr 54(3):374-80
3. Haas K, Rubesova E, Bass D(2016) Role of imaging in the evaluation of inflammatory bowel disease: How much is too much? World J Radiol 28;8(2):124-31
4. Levine A, Koletzko S, Turner D et al and European Society of Pediatric Gastroenterology, Hepatology, and Nutrition (2014) ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents J Pediatr Gastroenterol Nutr 58(6):795-80
5. Kaplan GG. (2015) The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 12(12):720-7.
6. Mathews JD, Forsythe AV, Brady Z et al (2013) Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ 21;346:f2360
7. Moy MP, Kaplan JL, Moran CJ et al (2016) MR Enterographic Findings as Biomarkers of Mucosal Healing in Young Patients With Crohn Disease. AJR Am J Roentgenol 28:1-7. [Epub ahead of print]
8. Moy MP, Sauk J, Gee MS (2016) The Role of MR Enterography in Assessing Crohn's Disease Activity and Treatment Response. Gastroenterol Res Pract. 2016:8168695.
9. Barber JL, Lozinsky AC, Kiparissi F et al (2016) Detecting inflammation in the unprepared pediatric colon - how reliable is magnetic resonance enterography? Pediatr Radiol 46(5):646-52.
10. Griffin N, Westerland O (2016) The Role of Magnetic Resonance Enterography in the Evaluation of Non-Crohn?s Pathologies. Semin Ultrasound CT MR 37(4):292-300.
11. Greer ML (2016) How we do it: MR enterography. Pediatr Radiol 46(6):818-28.
12. Rimola J, Planell N, Rodríguez S et al (2015) Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 110(3):432-40.
13. Li XH, Sun CH, Mao R et al (2017) Diffusion-weighted MRI Enables to Accurately Grade Inflammatory Activity in Patients of Ileocolonic Crohn's Disease: Results from an Observational Study. Inflamm Bowel Dis. 23(2):244-253.
14. AlSabban Z, Church P, Moineddin R (2017) Accuracy and interobserver agreement of diffusion-weighted imaging in pediatric inflammatory bowel disease. Clin Imaging 41:14-22.
Figures
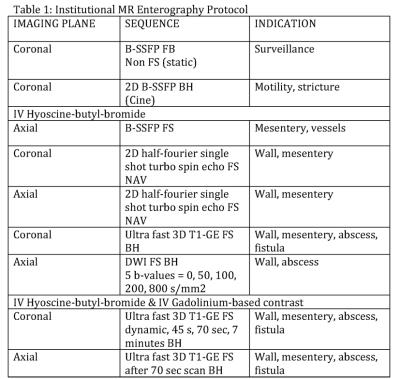
Figure 1
Abbreviations: FB – free breathing, BH – breath-hold, NAV – respiratory navigation, B-SSFP - balanced steady-state free precession, FS - fat suppressed, 2/3D - 2 or 3 dimensional, IV intravenous, GE – gradient echo, DWI – diffusion weighted imaging