DCE/DSC with Multiple Echoes: Blurring the Boundaries
1Department of Imaging Research, Barrow Neurological Institute
Synopsis
Contrast-enhanced MRI methods follow the dynamic passage of exogenous paramagnetic contrast agents to provide perfusion-related parameters, such as cerebral blood volume and cerebral blood flow, or permeability-related parameters, such as the volume transfer constant or extravascular extracellular volume. Perfusion- and permeability-related biomarkers can inform on different, but complementary, aspects related to vascular proliferation and angiogenic processes. Separate acquisitions and contrast injections are typically used to acquire both perfusion (DSC) and permeability (DCE) in patients. More advanced acquisitions involving multiple echoes permit simultaneous assessment of both perfusion and permeability information and may provide new insight into tumor-induced hemodynamic changes.
Highlights
- Perfusion- and permeability-related biomarkers can inform on different, but complementary, aspects related to vascular proliferation and angiogenic processes
- Perfusion is typically assessed with T2*-weighted signals using dynamic susceptibility contrast (DSC) MRI; permeability is typically assessed with T1-weighted signals using dynamic contrast-enhanced (DCE) MRI
- Separate acquisitions and contrast injections are typically used to acquire both DSC and DCE in patients
- More advanced acquisitions involving multiple echoes permit simultaneous assessment of both perfusion and permeability information and may provide new insight into tumor-induced hemodynamic changes
Outcome / Objectives
- Understand the similarities and differences between DSC- and DCE-MRI
- Understand the advantages and potential trade-offs for combined DSC- and DCE-MRI data acquisition
Introduction
Contrast-enhanced MRI (CE-MRI) methods follow the dynamic passage of exogenous paramagnetic contrast agents to provide perfusion-related parameters, such as cerebral blood volume (CBV) and cerebral blood flow (CBF), or permeability-related parameters, such as the volume transfer constant (Ktrans) or extravascular extracellular volume (ve). These parameters are widely used in cancer imaging to assess altered vascular characteristics and angiogenesis. While both CBV and Ktrans are often cited for assessing response to anti-angiogenic treatment, they may provide different but complementary information on angiogenesis (1). However, the calculation of both CBV and Ktrans typically requires two different scans (as well as two contrast injections). The combination of perfusion and permeability into a single acquisition may form a more complete basis for physiologic analysis of the complex and heterogeneous cancer microenvironment.
Two main categories of CE-MRI methods exist: perfusion-related parameters are assessed using Dynamic Susceptibility Contrast (DSC)-MRI methods that are predominately sensitive to dynamic T2 and/or T2* changes, while permeability-related parameters are assessed using Dynamic Contrast Enhanced (DCE)-MRI methods that are predominately sensitive to T1 changes. DSC-MRI methods rely on the contrast agent (CA) confinement to the intravascular space to induce strong susceptibility effects, while DCE-MRI methods rely on CA extravasation to induce T1 relaxation effects in the interstitial space. However, both methods can be adversely impacted by competing relaxation effects; specifically, T1 leakage effects can prevent reliable estimation of perfusion metrics in DSC-MRI (2), and vascular T2* effects can impact quantification of permeability metrics in DCE-MRI (3). While DSC-MRI perfusion metrics and DCE-MRI permeability metrics may individually benefit from the removal of undesirable T1 (in DSC-MRI) and T2* (in DCE-MRI) effects, the ideal sequence would permit quantification of both perfusion and permeability-related parameters. By modifying the pulse sequence to acquire multiple echoes, T1 and T2* effects can be effectively separated, enabling estimation of both perfusion and permeability-related parameters in a single acquisition.
Multi-echo acquisition methods
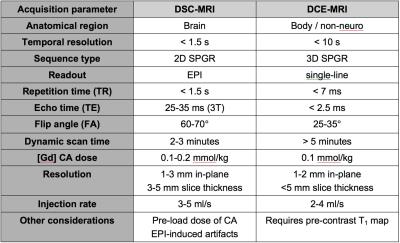
A dual-echo (or multi-echo) sequence can provide a wider dynamic range of T1 and T2* sensitivities. However, combining DSC and DCE-MRI into a single acquisition involves trade-offs between conflicting demands (Table 1). In particular, achieving sufficient T1-weighting for adequate concentration sensitivity can be problematic with a DSC-MRI EPI sequence, but achieving adequate temporal and spatial resolution can be problematic with DCE-MRI single-line acquisitions. Early dual-echo implementations involved single-line acquisitions with single slice coverage (4,5). In order to achieve the desired temporal and spatial resolutions, keyhole (4), sliding window (6), or interleaved acquisition methods (7,8) were often utilized. Later dual-echo (and multi-echo) implementations were developed using multi-slice multi-shot EPI, often in combination with parallel imaging to provide adequate scan parameters (2,9,10). Further improvements in temporal and/or spatial resolution may also be achieved through the use of non-Cartesian acquisition strategies (11), multiband excitation (12), and compressed sensing (13). Finally, further advancements in pulse sequence design have led to the ability to simultaneously measure T2*, T2, and T1 changes using a combined spin- and gradient-echo (SAGE) EPI sequence (14-19). These advancements have enabled the assessment of multiple complex tumor-related features, including vascular and microvascular flow and volume, cellularity, vessel size and architecture, and permeability.
Comparisons between conventional and multi-echo acquisitions have generally been promising. Quarles et al. (20) showed excellent correlation for DCE-MRI metrics of Ktrans and ve between a conventional single-echo acquisition and a multi-echo acquisition. Schmainda et al. (21) found no significant differences between spiral multi-echo rCBV and conventional EPI single-echo rCBV. In addition to the combined assessment of perfusion and permeability, another advantage to combined DSC-/DCE-MRI is the potential to reduce overall contrast agent dose. Overall, a combined approach leverages the advantages of both DSC-MRI and DCE-MRI to provide comprehensive information about tumors, both within the brain (where DSC is typically preferred) and outside of the brain (where DCE is typically preferred). While most of the applications thus far have been in brain tumors, other applications have included abdominal (8), breast (22), and prostate cancer (7).
Acknowledgements
Funding support from NIH/NCI 2R01CA158079.References
- Law M, Yang S, Babb JS, et al. Comparison of Cerebral Blood Volume and Vascular Permeability from Dynamic Susceptibility Contrast-Enhanced Perfusion MR Imaging with Glioma Grade. AJNR American journal of neuroradiology 2004;25:746-755.
- Vonken E-jPA, van Osch MJP, Bakker CJG, Viergever MA. Simultaneous quantitative cerebral perfusion and Gd-DTPA extravasation measurement with dual-echo dynamic susceptibility contrast MRI. Magn Reson Med 2000;43(6):820-827.
- Ewing JR, Bagher-Ebadian H. Model selection in measures of vascular parameters using dynamic contrast-enhanced MRI: experimental and clinical applications. Nmr Biomed 2013;26(8):1028-1041.
- Miyati T, Banno T, Mase M, et al. Dual dynamic contrast-enhanced MR imaging. Journal of Magnetic Resonance Imaging 1997;7(1):230-235.
- Barbier EL, den Boer JA, Peters AR, Rozeboom AR, Sau J, Bonmartin A. A model of the dual effect of gadopentetate dimeglumine on dynamic brain MR images. Journal of Magnetic Resonance Imaging 1999;10(3):242-253.
- d'Arcy JA, Collins DJ, Rowland IJ, Padhani AR, Leach MO. Applications of sliding window reconstruction with cartesian sampling for dynamic contrast enhanced MRI. Nmr Biomed 2002;15(2):174-183.
- Prochnow D, Beyersdorff D, Warmuth C, Taupitz M, Gemeinhardt O, Ludemann L. Implementation of a rapid inversion-prepared dual-contrast gradient echo sequence for quantitative dynamic contrast-enhanced magnetic resonance imaging of the human prostate. Magn Reson Imaging 2005;23(10):983-990.
- de Bazelaire C, Rofsky NM, Duhamel G, et al. Combined T2* and T1 measurements for improved perfusion and permeability studies in high field using dynamic contrast enhancement. European radiology 2006;16(9):2083-2091.
- Newbould RD, Skare ST, Jochimsen TH, et al. Perfusion mapping with multiecho multishot parallel imaging EPI. Magn Reson Med 2007;58(1):70-81.
- Jochimsen TH, Newbould RD, Skare ST, et al. Identifying systematic errors in quantitative dynamic-susceptibility contrast perfusion imaging by high-resolution multi-echo parallel EPI. Nmr Biomed 2007;20(4):429-438.
- Paulson ES, Prah DE, Schmainda KM. Spiral Perfusion Imaging With Consecutive Echoes (SPICE) for the Simultaneous Mapping of DSC- and DCE-MRI Parameters in Brain Tumor Patients: Theory and Initial Feasibility. Tomography : a journal for imaging research 2016;2(4):295-307.
- Barth M, Breuer F, Koopmans PJ, Norris DG, Poser BA. Simultaneous multislice (SMS) imaging techniques. Magn Reson Med 2016;75(1):63-81.
- Smith DS, Li X, Gambrell JV, et al. Robustness of Quantitative Compressive Sensing MRI: The Effect of Random Undersampling Patterns on Derived Parameters for DCE- and DSC-MRI. IEEE Transactions on Medical Imaging 2012;31(2):504-511.
- Schmiedeskamp H, Andre JB, Straka M, et al. Simultaneous perfusion and permeability measurements using combined spin- and gradient-echo MRI. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2013;33(5):732-743.
- Schmiedeskamp H, Straka M, Newbould RD, et al. Combined spin- and gradient-echo perfusion-weighted imaging. Magn Reson Med 2012;68(1):30-40.
- Skinner JT, Robison RK, Elder CP, Newton AT, Damon BM, Quarles CC. Evaluation of a multiple spin- and gradient-echo (SAGE) EPI acquisition with SENSE acceleration: applications for perfusion imaging in and outside the brain. Magn Reson Imaging 2014;32(10):1171-1180.
- Stokes AM, Quarles CC. A simplified spin and gradient echo approach for brain tumor perfusion imaging. Magn Reson Med 2016;75(1):356-362.
- Stokes AM, Skinner JT, Quarles CC. Assessment of a combined spin- and gradient-echo (SAGE) DSC-MRI method for preclinical neuroimaging. Magn Reson Imaging 2014;32(10):1181-1190.
- Stokes AM, Skinner JT, Yankeelov T, Quarles CC. Assessment of a simplified spin and gradient echo (sSAGE) approach for human brain tumor perfusion imaging. Magn Reson Imaging 2016;34(9):1248-1255.
- Quarles CC, Gore JC, Xu L, Yankeelov TE. Comparison of dual-echo DSC-MRI- and DCE-MRI-derived contrast agent kinetic parameters. Magn Reson Imaging 2012;30(7):944-953.
- Schmainda KM, Prah M, Baxter LC, et al. Simultaneous Measurement of DSC- and DCE-MRI Parameters using Dual-Echo Spiral with a Standard Dose of Gadolinium in Comparison to Single-Echo GRE-EPI Methods in Brain Tumors. Proceedings of the 23rd Annual Meeting of ISMRM, Proceedings of the 23rd Annual Meeting of ISMRM. Toronto, Ontario, Canada; 2015. p. 0487.
- Kuperman VY, Alley MT. Differentiation between the effects of T1 and T2* shortening in contrast-enhanced MRI of the breast. Journal of Magnetic Resonance Imaging 1999;9(2):172-176.
- Welker K, Boxerman J, Kalnin A, et al. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR American journal of neuroradiology 2015;36(6):E41-51.
- DCE MRI Quantification Profile. Quantitative Imaging Biomarkers Alliance. Available from: http://rsna.org/QIBA_.aspx: DCE MRI Technical Committee; 2012.