Motion Compensation: Pulse Sequence & Reconstruction Strategies
1Imaging Sciences and Biomedical Engineering, King's College London, London, United Kingdom, 2Escuela de Ingeniería, Pontificia Universidad Católica de Chile, Santiago de Chile, Chile
Synopsis
Over the past decade Magnetic Resonance Imaging (MRI) has become an increasingly important non-invasive tool in risk assessment and treatment monitoring of cardiovascular disease. However, despite ongoing progress and developments in MR acquisition and reconstruction technology, physiological motion remains a major problem in many cardiovascular MRI applications. Since MR acquisition is slow compared to physiological motion, the extensive cardiac and respiratory induced motion of the heart during the acquisition period can degrade image quality by introducing ghosting and blurring like motion artifacts. Several cardiac and respiratory motion compensation techniques have been proposed over the last two decades to overcome this problem. These techniques are based on minimizing or correcting the motion during the acquisition. This part of the Image Acquisition & Reconstruction Course at ISMRM 2017 will include an overview of some of these methods, discussing their strengths and limitations.
Highlights
- Motion can be corrected for prospectively or retrospectively
- Motion sensing is either performed with external sensors, MR navigators or self-navigation (data itself)
- Motion correction can be subdivided into translational, affine and non-rigid, which can be applied in a step wise fashion with increasing computational complexity
- Gating can be used to minimize the accepted motion range or do discard outliers but prolongs scan time
- Instead of correcting for motion an alternative approach is to resolve motion states, which is usually achieved with a multi dimensional compressed sensing reconstruction
Target Audience
Physicists and engineers who wish to acquire an understanding of how cardiac and respiratory motion can be measured and corrected for.Educational Objectives
To better understand current motion sensitization and compensation techniquesAbstract
Purpose
Over the past decade Magnetic Resonance Imaging (MRI) has become an increasingly important non-invasive tool in risk assessment and treatment monitoring of cardiovascular disease. However, despite ongoing progress and developments in MR acquisition and reconstruction technology, physiological motion remains a major problem in many cardiovascular MRI applications. Since MR acquisition is slow compared to physiological motion, the extensive cardiac and respiratory induced motion of the heart during the acquisition period can degrade image quality by introducing ghosting and blurring like motion artifacts. Several cardiac and respiratory motion compensation techniques have been proposed over the last two decades to overcome this problem. These techniques are based on minimizing or correcting the motion during the acquisition. This part of the Image Acquisition & Reconstruction Course at ISMRM 2017 will include an overview of some of these methods, discussing their strengths and limitations.
Cardiac-induced Motion
In order to minimize cardiac motion data have to be acquired within a time frame (acquisition window) which is short in comparison to the cardiac cycle length. In the majority of cardio-vascular MR acquisitions data from the same cardiac phase but different cardiac cycles are acquired during a short acquisition window and then combined under the (strong) assumption that cardiac motion is the same between heartbeats (segmented acquisition). Image data are acquired either in a single phase of the cardiac cycle using prospective cardiac triggering or in multiple phases (CINE MRI) using cardiac gating. Cardiac triggering is mainly used for high-resolution anatomical images, as coronary MR angiography and late-gadolinium enhancement. With this approach data acquisition is carried out within a short acquisition window during a quiescent period in the cardiac cycle (i.e. end-systole or mid-diastole). Cardiac gating (prospectively or retrospectively) is mainly used for CINE MRI and data is acquired throughout the entire cardiac cycle. Both approaches require information about the cardiac cycles for cardiac synchronization.
Information about cardiac cycles is commonly obtained from external electrocardiogram (ECG) devices. The ECG signal represents the electrical activity of the heart muscle which correlates with cardiac contraction and relaxation. The R-peak of the ECG signal is detected and used as reference point during MRI acquisition. However, a reliable signal for cardiac synchronization is not always available with this approach since the quality of ECG can be strongly impaired by several sources of noise (gradient and RF fields) and the magneto-hydrodynamic effect (especially in high and ultrahigh field scanners). Furthermore, in some applications such as fetal imaging an external ECG device is not applicable [1].
Cardiac synchronization may be also achieved by using acoustic triggering techniques that exploit heart sounds created by the opening and closing of the heart valves [2] or pulse oximeters [3]. However, both approaches require dedicated devices and additional hardware and may suffer from limited accuracy. Cardiac self-gating approaches have been proposed to overcome some of these problems by estimating the cardiac-induced motion from the acquired data itself. These methods usually acquire the central k-space line repeatedly during the imaging sequence to derive an ECG-like signal [4-8]. Most of these techniques rely on changes of the overall signal intensity of the entire field-of-view (FOV) mainly due to increases and decreases in blood volume during the cardiac cycle. A limitation of these approaches is that they cannot accurately distinguish between changes of the blood pool due to cardiac motion and changes due to other effects, which also lead to signal variations, such as in flow effects. Recently an image-based cardiac navigator signal derived from the motion of the left ventricle during the cardiac cycle has been proposed [9]. This approach uses 2D real-time images obtained with a golden angle radial sampling scheme to obtain a cardiac gating signal directly from physiological processes of the heart.
Respiratory-induced Motion
Normal breathing leads to a shift and deformation of the heart, primarily in the foot-head (FH) direction [10] but also leads to additional 3D affine and non-linear motion components that differ strongly between different subjects [11-14].
A simple approach to minimize respiratory-induced motion artefacts is to perform the acquisition during one or multiple breathholds (~15-20s). However, this approach is incompatible with clinically preferred high-resolution 3D targeted or 3D whole-heart coronary MRI due to the long scan time required to satisfy signal-to-noise and spatial resolution requirements. Similar to cardiac motion minimization, respiratory motion monitoring (or surrogates) can be used in free breathing 3D coronary MRI to combine data from multiple breathing cycles acquired at a similar respiratory position. Surrogates are used to relate the chest wall or diaphragmatic motion directly to the respiratory motion of the heart. Systems to monitor the motion of the chest wall include external devices such as pneumatic respiratory bellows [15,16], while techniques to monitor the hemi-diaphragm position include the commonly used one-dimensional (1D) navigator echoes [17].
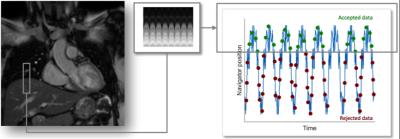
A navigator echo acquires signal from a long narrow volume oriented along the FH direction to monitor the position of the lung-liver (right hemi-diaphragm) interface. This 1D navigator signal can be achieved using two dimensional (2D)-selective RF pulses (pencil-beam navigator)[18,19] or using a spin echo technique with obliquely aligned excitation and refocusing planes, so that signal is only acquired from the intersection of the two planes [17,20]. In both methods a single line of k-space is sampled so that Fourier reconstruction yields a projection of the volume onto the FH direction. Therefore, the repetitive acquisition of navigator echoes provides continuous monitoring of the diaphragmatic FH translation as shown in Figure 1. The respiratory signal provided by the navigator echoes is used to gate the acquisition. Using gating, data are accepted/acquired only when the respiratory signal is within a predefined acceptance window of the breathing cycle (typically end-expiration) with all other data being rejected (Figure 1). Rejected data have to be reacquired in the subsequent cardiac cycles. This approach has shown to considerably reduce motion artifacts when small gating windows are employed (3-5mm), however progressively smaller gating windows lead to more prolonged scan times since a smaller fraction of the acquired data are then accepted for reconstruction (referred to as scan efficiency). For example a high-resolution 3D scan with a nominal scan time of 5-6 minutes may take 10-20 minutes for a typical scan efficiency of ~25-50%. Moreover, in subjects with highly irregular breathing patterns drift in respiratory motion can lead to scan abortions due to zero gating efficiency.
1D navigators can be also used to correct for residual motion within the gating window, an approach usually referred as gate and track. This method assumes that the heart motion is dominated by translation in the FH direction, and that this displacement is proportional to that of the diaphragm [10]. The measured displacement at the diaphragm can be used to correct for motion within the acceptance window by prospectively adapting the position of the imaging slice to the measured respiratory position (slice tracking) or by retrospectively applying a linear phase to the k-space data. The proportional scaling factor between the diaphragmatic and cardiac displacements is known as the tracking factor. Commonly a fixed scaling factor of 0.6, as suggested by Wang et al. [10], is used for slice tracking, however, additional methods have been proposed to derive subject-specific correction factors [21,22]. This correction method is however limited because it does not measure directly the motion of the heart, and does not account for non-linearities including hysteresis effects between inspiration and expiration [13]. Furthermore, neither more complex multidimensional rotational, affine or non-linear motion components are included.
Self-navigation methods have been proposed to derive the respiratory-induced motion of the heart from the acquired data itself without the need of either a 1D navigator echo or a heart-diaphragm tracking factor. Respiratory-induced displacements of the heart can be directly estimated from the repetitive acquisition of the central k-space point [23] or the central k-space line [24-27], corresponding to zero-dimensional or one-dimensional projections of the field of view. Similar to the 1D navigator echo approaches self-navigation methods typically perform motion correction only in the FH translational motion direction. Another drawback of the 1D self-navigation approach is the inclusion of static structures, such as the chest wall, which can degrade motion estimation and correction.
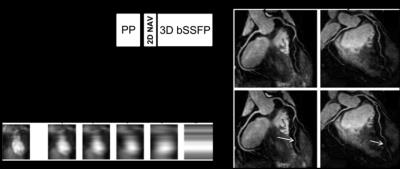
To overcome these problems and account for more complex motion, several 2D and 3D image-based navigator (iNAV) approaches have recently been proposed for coronary MRI [28-35]. In these approaches a low-resolution 2D or 3D image is acquired in every heartbeat before (or after) the coronary MRI data (Figure 2). The main advantage of this approach is that the moving heart can be spatially isolated from surrounding static tissues and the respiratory-induced cardiac motion can be directly estimated via (rigid or affine) image-registration of iNAVs at different respiratory positions.
Self-navigation and image-based navigator methods can be used to gate the acquisition and correct for motion within a small gating window as described for 1D navigator echoes. Furthermore, since these methods directly track heart motion/position, a much larger gating window can be used, or it can be removed entirely, thereby increasing the scan efficiency to or close to 100%. These promising approaches may lead to shorter and predictable scan times, as described in the next section.
Acquisition Speed
For several cardiac MR examinations (e.g. coronary MRI, late gadolinium enhancement (LGE)) high isotropic spatial resolution is required, ideally around 1mm, to correctly visualize and characterize small pathologies and the coronary arteries and plaque. This results in long MRI acquisition times.
Several approaches have been proposed to accelerate data acquisition including fast trajectories, undersampling reconstruction techniques and motion correction approaches with 100% scan efficiency. Advantages of fast trajectories, including spiral and radial acquisitions, are more efficient filling of k-space, incoherent undersampling and decreased sensitivity to motion [36-40]. At present these technical approaches have not yet become established standards for e.g. coronary MRI or LGE due to their off-resonance sensitivity (spiral) or signal-to-noise ratio penalty (radial). However 3D radial acquisitions combined with self-gating signals have recently been demonstrated to achieve 100% scan efficiency with promising initial results [41-43]. Moreover 3D radial acquisitions have been used to reconstruct coronary MR images at multiple cardiac phases (four-dimensional, 4D, reconstruction), enabling simultaneous left ventricular functional assessment and whole-heart coronary artery visualization from a single free-running scan [44,45]. Example results of 4D radial coronary MR angiography are shown in Figure 2. A similar approach has been used to reconstruct several respiratory phases for a single cardiac phase. This has been achieved using an extradimensional golden-angle radial sparse parallel imaging (XD-GRASP) algorithm, which exploits sparsity along the respiratory dimension. [46]
Parallel imaging reconstruction techniques such as SENSE or GRAPPA [47-50] with acceleration factors of 1.5 to 2 times have become the standard to reduce the acquisition time in coronary MR acquisitions while maintaining image quality. Further acceleration may be achieved with compressed sensing techniques [51,52] although its efficacy in clinical practice has yet to be established.
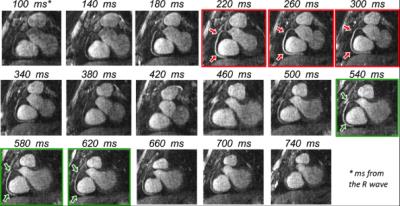
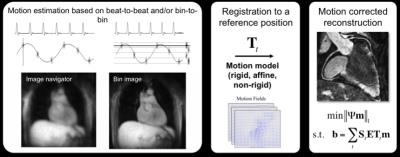
Self-navigation and image-based navigator methods have been proposed to accelerate the scan to achieve ~100% scan efficiency. This is accomplished by acquiring data during free breathing with no, or minimal rejection, and correcting for respiratory motion. Most of these approaches correct respiratory motion in a beat-to-beat fashion, however they are typically limited to 1D, 2D or 3D translational motion only. To account for more complex affine and non-linear motion, ‘respiratory binning’ techniques have been proposed recently [41,42,53-57]. With these approaches, a 1D respiratory signal is used to divide the coronary MRI data into several states of the breathing cycle or respiratory “bins”. Images reconstructed at each respiratory bin are then registered to estimate the motion and correction for coronary motion is performed in a bin-to-bin fashion. Motion correction can be performed directly in k-space (for rigid and affine motion), incorporated into the reconstruction process (for general non-linear motion) or can be achieved by averaging images from different motion states after warping them to a common reference position (to reduce computational costs). A schematic of a ~100% scan efficiency motion correction framework is shown in Figure 3. Example results of a combined iNAV-based beat-to-beat 2D translational and respiratory bin-to-bin 3D non-linear motion correction approach are shown in Figure 4 for coronary MR angiography. These methods show promising results in healthy subjects and have the potential to be combined with undersampled reconstruction techniques to further accelerate the acquisition. An alternative approach to nonlinear motion correction has also been introduced in the form of localized autofocusing techniques [28,32,58]. These novel technical developments are currently mainly in the proof-of-concept state and clinical validation is awaited to establish their clinical efficacy.
This talk will include examples of the above methods for cardiac and respiratory motion compensation, discussing their strengths and limitations. An extensive review in motion in cardiovascular MR imaging can be found in [59,60].
Acknowledgements
The authors acknowledge financial support from the British Heart Foundation (RG/12/1/29262), the BHF Centre of Excellence (RE/08/03), the EPSRC (EP/P001009/1 and EP/P007619/1), FONDECYT N° 1161051 and CONICYT-ANILLO ACT 1416. The Division of Imaging Sciences receives also support from the Centre of Excellence in Medical Engineering (funded by the Welcome Trust and EPSRC; grant number WT 088641/Z/09/Z) and the Department of Health through the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King’s College Hospital NHS Foundation Trust, and by the NIHR Healthcare Technology Co-operative for Cardiovascular Disease at Guy’s and St Thomas’ NHS Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Yamamura, J.; Kopp, I.; Frisch, M., et al., Cardiac mri of the fetal heart using a novel triggering method: Initial results in an animal model. Journal of magnetic resonance imaging : JMRI 2012, 35, 1071-6.
2. Becker, M.; Frauenrath, T.; Hezel, F., et al., Comparison of left ventricular function assessment using phonocardiogram- and electrocardiogram-triggered 2d ssfp cine mr imaging at 1.5 t and 3.0 t. European radiology 2010, 20, 1344-55.
3. Denslow, S.; Buckles, D.S., Pulse oximetry-gated acquisition of cardiac mr images in patients with congenital cardiac abnormalities. AJR. American journal of roentgenology 1993, 160, 831-3.
4. Spraggins, T.A., Wireless retrospective gating: Application to cine cardiac imaging. Magnetic resonance imaging 1990, 8, 675-81.
5. Larson, A.C.; White, R.D.; Laub, G., et al., Self-gated cardiac cine mri. Magnetic resonance in medicine 2004, 51, 93-102.
6. Crowe, M.E.; Larson, A.C.; Zhang, Q., et al., Automated rectilinear self-gated cardiac cine imaging. Magnetic resonance in medicine 2004, 52, 782-8.
7. Nijm, G.M.; Sahakian, A.V.; Swiryn, S., et al., Comparison of self-gated cine mri retrospective cardiac synchronization algorithms. Journal of magnetic resonance imaging : JMRI 2008, 28, 767-72.
8. Yamamura, J.; Frisch, M.; Ecker, H., et al., Self-gating mr imaging of the fetal heart: Comparison with real cardiac triggering. European radiology 2011, 21, 142-9.
9. Kolbitsch, C.; Prieto, C.; Buerger, C., et al., Prospective high-resolution respiratory-resolved whole-heart mri for image-guided cardiovascular interventions. Magnetic resonance in medicine 2012, 68, 205-13.
10. Wang, Y.; Riederer, S.J.; Ehman, R.L., Respiratory motion of the heart: Kinematics and the implications for the spatial resolution in coronary imaging. Magn Reson Med 1995, 33, 713-9.
11. Danias, P.G.; Stuber, M.; Botnar, R.M., et al., Relationship between motion of coronary arteries and diaphragm during free breathing: Lessons from real-time mr imaging. AJR. American journal of roentgenology 1999, 172, 1061-5.
12. McLeish, K.; Hill, D.L.; Atkinson, D., et al., A study of the motion and deformation of the heart due to respiration. IEEE transactions on medical imaging 2002, 21, 1142-50.
13. Nehrke, K.; Bornert, P.; Manke, D., et al., Free-breathing cardiac mr imaging: Study of implications of respiratory motion--initial results. Radiology 2001, 220, 810-5.
14. Shechter, G.; Ozturk, C.; Resar, J.R., et al., Respiratory motion of the heart from free breathing coronary angiograms. IEEE transactions on medical imaging 2004, 23, 1046-56.
15. McConnell, M.V.; Khasgiwala, V.C.; Savord, B.J., et al., Comparison of respiratory suppression methods and navigator locations for mr coronary angiography. AJR Am J Roentgenol 1997, 168, 1369-75.
16. Santelli, C.; Nezafat, R.; Goddu, B., et al., Respiratory bellows revisited for motion compensation: Preliminary experience for cardiovascular mr. Magnetic resonance in medicine 2011, 65, 1097-102.
17. Ehman, R.L.; Felmlee, J.P., Adaptive technique for high-definition mr imaging of moving structures. Radiology 1989, 173, 255-63.
18. Danias, P.G.; McConnell, M.V.; Khasgiwala, V.C., et al., Prospective navigator correction of image position for coronary mr angiography. Radiology 1997, 203, 733-6.
19. Nehrke, K.; Bornert, P.; Groen, J., et al., On the performance and accuracy of 2d navigator pulses. Magnetic resonance imaging 1999, 17, 1173-81.
20. Firmin, D.; Keegan, J., Navigator echoes in cardiac magnetic resonance. J Cardiovasc Magn Reson 2001, 3, 183-93.
21. Moghari, M.H.; Hu, P.; Kissinger, K.V., et al., Subject-specific estimation of respiratory navigator tracking factor for free-breathing cardiovascular mr. Magnetic resonance in medicine 2012, 67, 1665-72.
22. Taylor, A.M.; Keegan, J.; Jhooti, P., et al., Calculation of a subject-specific adaptive motion-correction factor for improved real-time navigator echo-gated magnetic resonance coronary angiography. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 1999, 1, 131-8.
23. Buehrer, M.; Curcic, J.; Boesiger, P., et al., Prospective self-gating for simultaneous compensation of cardiac and respiratory motion. Magnetic resonance in medicine 2008, 60, 683-90.
24. Kim, W.S.; Mun, C.W.; Kim, D.J., et al., Extraction of cardiac and respiratory motion cycles by use of projection data and its applications to nmr imaging. Magnetic resonance in medicine 1990, 13, 25-37.
25. Lai, P.; Bi, X.; Jerecic, R., et al., A respiratory self-gating technique with 3d-translation compensation for free-breathing whole-heart coronary mra. Magnetic resonance in medicine 2009, 62, 731-8.
26. Piccini, D.; Littmann, A.; Nielles-Vallespin, S., et al., Respiratory self-navigation for whole-heart bright-blood coronary mri: Methods for robust isolation and automatic segmentation of the blood pool. Magn Reson Med 2012, 68, 571-9.
27. Stehning, C.; Bornert, P.; Nehrke, K., et al., Free-breathing whole-heart coronary mra with 3d radial ssfp and self-navigated image reconstruction. Magnetic resonance in medicine 2005, 54, 476-80.
28. Addy, N.O.; Ingle, R.R.; Luo, J., et al., 3d image-based navigators for coronary mr angiography. Magnetic resonance in medicine 2016.
29. Henningsson, M.; Koken, P.; Stehning, C., et al., Whole-heart coronary mr angiography with 2d self-navigated image reconstruction. Magnetic resonance in medicine 2012, 67, 437-45.
30. Henningsson, M.; Smink, J.; Razavi, R., et al., Prospective respiratory motion correction for coronary mr angiography using a 2d image navigator. Magnetic resonance in medicine 2013, 69, 486-94.
31. Kawaji, K.; Spincemaille, P.; Nguyen, T.D., et al., Direct coronary motion extraction from a 2d fat image navigator for prospectively gated coronary mr angiography. Magnetic resonance in medicine 2014, 71, 599-607.
32. Luo, J.; Addy, N.O.; Ingle, R.R., et al., Nonrigid motion correction with 3d image-based navigators for coronary mr angiography. Magnetic resonance in medicine 2016.
33. Moghari, M.H.; Annese, D.; Geva, T., et al., Three-dimensional heart locator and compressed sensing for whole-heart mr angiography. Magnetic resonance in medicine 2016, 75, 2086-93.
34. Moghari, M.H.; Roujol, S.; Henningsson, M., et al., Three-dimensional heart locator for whole-heart coronary magnetic resonance angiography. Magn Reson Med 2014, 71, 2118-26.
35. Wu, H.H.; Gurney, P.T.; Hu, B.S., et al., Free-breathing multiphase whole-heart coronary mr angiography using image-based navigators and three-dimensional cones imaging. Magnetic resonance in medicine 2013, 69, 1083-93.
36. Bornert, P.; Aldefeld, B.; Nehrke, K., Improved 3d spiral imaging for coronary mr angiography. Magnetic resonance in medicine 2001, 45, 172-5.
37. Bornert, P.; Stuber, M.; Botnar, R.M., et al., Direct comparison of 3d spiral vs. Cartesian gradient-echo coronary magnetic resonance angiography. Magnetic resonance in medicine 2001, 46, 789-94.
38. Jahnke, C.; Paetsch, I.; Schnackenburg, B., et al., Comparison of radial and cartesian imaging techniques for mr coronary angiography. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 2004, 6, 865-75.
39. Priest, A.N.; Bansmann, P.M.; Mullerleile, K., et al., Coronary vessel-wall and lumen imaging using radial k-space acquisition with mri at 3 tesla. European radiology 2007, 17, 339-46.
40. Spuentrup, E.; Katoh, M.; Buecker, A., et al., Free-breathing 3d steady-state free precession coronary mr angiography with radial k-space sampling: Comparison with cartesian k-space sampling and cartesian gradient-echo coronary mr angiography--pilot study. Radiology 2004, 231, 581-6.
41. Bhat, H.; Ge, L.; Nielles-Vallespin, S., et al., 3d radial sampling and 3d affine transform-based respiratory motion correction technique for free-breathing whole-heart coronary mra with 100% imaging efficiency. Magnetic resonance in medicine 2011, 65, 1269-77.
42. Pang, J.; Sharif, B.; Arsanjani, R., et al., Accelerated whole-heart coronary mra using motion-corrected sensitivity encoding with three-dimensional projection reconstruction. Magnetic resonance in medicine 2015, 73, 284-91.
43. Piccini, D.; Monney, P.; Sierro, C., et al., Respiratory self-navigated postcontrast whole-heart coronary mr angiography: Initial experience in patients. Radiology 2014, 270, 378-86.
44. Coppo, S.; Piccini, D.; Bonanno, G., et al., Free-running 4d whole-heart self-navigated golden angle mri: Initial results. Magnetic resonance in medicine 2015, 74, 1306-16.
45. Pang, J.; Sharif, B.; Fan, Z., et al., Ecg and navigator-free four-dimensional whole-heart coronary mra for simultaneous visualization of cardiac anatomy and function. Magn Reson Med 2014, 72, 1208-17.
46. Piccini, D.; Feng, L.; Bonanno, G., et al., Four-dimensional respiratory motion-resolved whole heart coronary mr angiography. Magnetic resonance in medicine 2017, 77, 1473-84.
47. Griswold, M.A.; Jakob, P.M.; Heidemann, R.M., et al., Generalized autocalibrating partially parallel acquisitions (grappa). Magnetic resonance in medicine 2002, 47, 1202-10.
48. Pruessmann, K.P.; Weiger, M.; Scheidegger, M.B., et al., Sense: Sensitivity encoding for fast mri. Magn Reson Med 1999, 42, 952-62.
49. Niendorf, T.; Hardy, C.J.; Giaquinto, R.O., et al., Toward single breath-hold whole-heart coverage coronary mra using highly accelerated parallel imaging with a 32-channel mr system. Magn Reson Med 2006, 56, 167-76.
50. Wielopolski, P.A.; van Geuns, R.J.; de Feyter, P.J., et al., Breath-hold coronary mr angiography with volume-targeted imaging. Radiology 1998, 209, 209-19.
51. Akcakaya, M.; Basha, T.A.; Chan, R.H., et al., Accelerated isotropic sub-millimeter whole-heart coronary mri: Compressed sensing versus parallel imaging. Magnetic resonance in medicine 2014, 71, 815-22.
52. Lustig, M.; Donoho, D.; Pauly, J.M., Sparse mri: The application of compressed sensing for rapid mr imaging. Magnetic resonance in medicine 2007, 58, 1182-95.
53. Pang, J.; Bhat, H.; Sharif, B., et al., Whole-heart coronary mra with 100% respiratory gating efficiency: Self-navigated three-dimensional retrospective image-based motion correction (trim). Magnetic resonance in medicine 2013.
54. Aitken, A.P.; Henningsson, M.; Botnar, R.M., et al., 100% efficient three-dimensional coronary mr angiography with two-dimensional beat-to-beat translational and bin-to-bin affine motion correction. Magn Reson Med 2015, 74, 756-64.
55. Cruz, G.; Atkinson, D.; Henningsson, M., et al., Highly efficient nonrigid motion-corrected 3d whole-heart coronary vessel wall imaging. Magn Reson Med 2016.
56. Henningsson, M.; Prieto, C.; Chiribiri, A., et al., Whole-heart coronary mra with 3d affine motion correction using 3d image-based navigation. Magnetic resonance in medicine 2014, 71, 173-81.
57. Prieto, C.; Doneva, M.; Usman, M., et al., Highly efficient respiratory motion compensated free-breathing coronary mra using golden-step cartesian acquisition. Journal of magnetic resonance imaging : JMRI 2015, 41, 738-46.
58. Ingle, R.R.; Wu, H.H.; Addy, N.O., et al., Nonrigid autofocus motion correction for coronary mr angiography with a 3d cones trajectory. Magnetic resonance in medicine 2014, 72, 347-61.
59. Scott, A.D.; Keegan, J.; Firmin, D.N., Motion in cardiovascular mr imaging. Radiology 2009, 250, 331-51.
60. Henningsson, M.; Botnar, R.M., Advanced respiratory motion compensation for coronary mr angiography. Sensors (Basel) 2013, 13, 6882-99.
Figures