Multi-Tuned Coils
1High-Field MR Center, Max Planck Institute for Biological Cybernetics, Tübingen, Germany, 2Institute of Physics, Ernst-Moritz-Arndt University of Greifswald, Greifswald, Germany
Synopsis
X-nuclei (13C, 31P, 19F etc) MRI and spectroscopy are of great interest since these methods provide a non-invasive technique to study in-vivo metabolite changes due to various diseases. To provide anatomical landmarks for interpretation of X-nuclei spectroscopic data, 1H anatomical images are required. To eliminate uncertainties associated with repositioning the patient, the RF coil must also resonate at the 1H frequency. This technique is called double-tuning (DT) of the RF coils. The choice of DT design is determined by the requirements of a specific application. Various methods of constructing DT RF surface coils, volume coils, and phased arrays are discussed.
Background
Target audience: RF engineers, scientists and students interested in development, construction and usage of double-tuned (DT) RF MRI coils for X-nuclei spectroscopic studies.
Purpose: To learn various techniques of DT RF coil design and construction
Highlights:
-Introduction
-Usefull RF circuits (traps) used for DT coil design
-DT surface coils
-DT Volume coils
-DT phased Arrays
Introduction
X-nuclei (13C, 31P, 19F, 23Na, 7Li etc) magnetic resonance imaging (MRI) and spectroscopy (MRS, MRSI) are of great interest to our scientific community since these methods provide a unique non-invasive technique to study in-vivo metabolite changes due to various diseases. To provide anatomical landmarks for interpretation of X-nuclei spectroscopic data, high-resolution 1H anatomical images are also required. To eliminate uncertainties associated with repositioning either the patient or the RF coil and to support 1H imaging, the RF coil must also resonate at the 1H frequency. This technique is often referred to as double-tuning of the MRI RF coils. B0 shimming and proton decoupling (e.g. 13C) also require double-tuning the X-nuclei coils. Various methods of constructing double-tuned (DT) RF coils have been previously described. The choice of DT design is always determined by the requirements of a specific application, e.g. whether both channels (1H, X) are used simultaneously, or the 1H-channel should have high sensitivity. Required field-of-views of both channels are also of great importance. X-nuclei MR signals are intrinsically lower due to a large difference in the gyromagnetic ratio. This implies that DT design is mostly optimized for X-nuclei performance. For the same reason, recent advances in ultra-high field (UHF, >7T) MRI, which provide for higher signal-to-noise ratio (SNR), increase interest in MRS studies.
The lecture starts with a discussion of useful basic RF circuits followed by an introduction of various basic methods of double-tuning surface RF coils [1-13], volume coils [14-30], and phased arrays [31-43]. Phased arrays are especially important at UHF.
Surface Coils
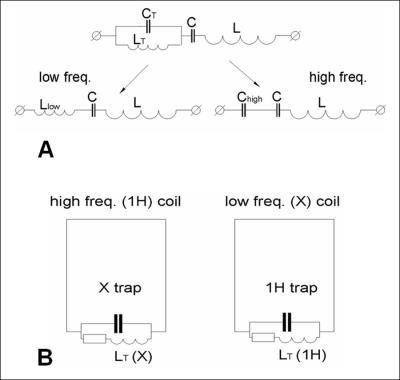
The most common way of constructing DT surface coils is by introducing a resonance LC-trap circuit in series with distributed capacitors. This is mathematically referred as pole insertion [1]. In this case, the same inductive loop of the surface coil, L, is used to generate the RF B1 field at both frequencies. A trap, formed by a parallel resonance circuit (LT, CT) is connected in series with a capacitor, C, and an inductor, L (Fig.1A). At low frequencies (ω2LTCT < 1), the trap’s impedance, ZT, is equivalent to an inductor, Llow, while at higher frequencies (ω2LTCT > 1) it acts as a capacitor, Chigh, thus, producing a double resonant circuit (Fig.1A). The performance of the surface coil at both frequencies can be estimated as E= [PL/(PL+PT)]1/2=[RL/(RL+RT)]1/2, where PL,T and RL,T are power losses and resistances associated with inductors L and LT, respectively. Efficiency at low, Elow, and high, Ehigh, frequencies can then be calculated using Eqs. (1) and (2) as obtained in [1].
Elow=(L/(L+LT))1/2 (1)
Ehigh=(LT/(L+LT))1/2 (2)
Using these equations, for LT = 0.2 L, we obtain an Elow of 0.91 and and Ehigh of 0.41. Thus, LC-trap design does not allow optimization of both channels in a DT coil simultaneously. While it provides a certain convenience in design, the coil always possesses substantially lower sensitivity at 1H-frequency. Various DT matching circuits are also discussed [13].
Another approach to construct a DT coil is by utilizing separate surface loops (or other types of RF coils) resonating at the X-nuclei and 1H-frequencies [2,4-8,10-12] as shown in Fig.1B. Despite a large difference in resonance frequencies, two coils can still strongly interact with one another. In order to cancel cross-talk, resonant traps are, therefore still inserted into each loop, i.e. an X-trap into the 1H -loop and vice versa. However, in this case, traps resonating at substantially different frequencies do not compromise the coil performance. Performance of both loops can thus be optimized simultaneously. Size and geometry of X- and 1H-coils are determined by the requirements of the specific experiment. Using a pair of geometrically decoupled coils also helps to eliminate a cross-talk between two channels of the DT coil [6,11]. Other options include using orthogonal modes of the same coil [12] or switching the resonance frequency using PIN diodes [9]. The last option, however, does not allow the use of both channels simultaneously.
Volume Coils
Optimal SNR for in vivo studies is typically obtained when a homogeneous volume coil is used for transmission and a sensitive local receive-only coil (phased array) is used for reception. In this setup, double-tuning of the transmit volume coil is often required. In this section examples of DT volume coils based on common multi-mode birdcage [1,2] and other designs are provided [14-30]. As with the double-tuning of surface coils, DT volume coils can be constructed using either the same coil resonating at X- and 1H-frequencies simultaneously [17,18,20-26,27,28] or two different nested volume coils [19,26]. Switching the frequency of a single-tuned birdcage coil using PIN diodes [30] may provide a solution when simultaneous use of both channels is not required.Phased Arrays
The improved signal-to-noise ratio (SNR) at UHF provides significant advantages for both 1H and lower gyromagnetic ratio X-nuclei. Double resonant volume coils based on both birdcage and TEM [21] designs have been previously used at lower magnetic fields. However, at UHF, phased arrays [31] provide significant advantages for transmit B1 homogeneity and efficiency over volume coils. Similarly, phased arrays for reception provide additional SNR gains for peripheral locations in studies of X nuclei. Therefore, DT transceiver arrays may provide substantial advantages over conventional DT volume coils. Nevertheless, DT transceiver arrays are substantially more complicated than single tuned arrays as all individual elements must be decoupled from each other at both resonance frequencies. In the last part of the presentation, several recent developments of DT phased arrays are discussed (32-42). This includes both transceiver DT arrays [33-37, 42], and Transmit-only/ Receive-only (ToRo) array combinations [32, 38-41].Acknowledgements
No acknowledgement found.References
REFERENCES
Books
J. Mispelter, M. Lupu, and A. Briguet, NMR Probeheads: for Biophysical and Biomedical Experiments, Imperial College Press: London, 2006.
Articles
Surface Coils
1. Schnall MD, Subramanian VH, Leigh JS and Chance B, A new double-tuned probed for concurrent 1H and 31P NMR, J Magn Res 1985; 65: 122-129.
2. Fitzsimmons JR, Brooker HR, Beck B, A transformer-coupled double-resonant probe for NMR imaging and spectroscopy, Magn Reson Med 1987; 5: 471-477.
3. Elhadi Najim E, Jean-Philippe Grivet J-P, Efficiency estimation for single-coil, separate-input, double-tuned NMR probes, J. Magn. Res 1991; 93: 27-33.
4. Fitzsimmons JR, Brooker HR, Beck B, A comparison of double-tuned surface coils, Magn Res Med 1989;10: 302-309.
5. Adriany G and Gruetter R, A Half-Volume Coil for Efficient Proton Decoupling in Humans at 4 Tesla, J Magn Res1997; 125: 178-184.
6. Klomp DWJ, Collins DJ, van den Boogert HJ, Schwarz A, Rijpkema Prock MT, Payne GS, Leach MO and Heerschap A, Radio-frequency probe for 1H decoupled 31P MRS of the head and neck region, Magn Res Imag 2001; 19: 755-759.
7. Alecci M, Romanzetti S, Kaffanke J, Celik A, Wegener HP, and Shah NJ, Practical design of a 4 Tesla double-tuned RF surface coil for interleaved 1H and 23Na MRI of rat brain. J Magn Reson 181(2):203-11, 2006.
8. Dabirzadeh A, McDougall MP, Trap design for insertable second-nuclei radiofrequency coils for magnetic resonance imaging and spectroscopy, Conc. Magn Reson B: Magn Reson Eng., 35B(3), 121-132, 2009.
9. Choi C-H, Hutchison JMS, Lurie DJ, Design and construction of an actively frequency-switchable RF coil for field-dependent Magnetisation Transfer Contrast MRI with fast field-cycling, J Magn Res 2010; 207: 134-139.
10. Roig ES, Magill AW, Donati G, Meyerspeer M, Xin L, Ipek O and Gruetter R, A double-quadrature radiofrequency coil design for proton-decoupled carbon-13 magnetic resonance spectroscopy in humans at 7T, Magn Reson Med 2015; 73: 894-900.
11. Rutledge O, Kwak T, Cao P, Zhang X, Design and test of a double-nuclear RF coil for 1H MRI and 13C MRSI at 7 T, J Magn Reson 2016; 267: 15-21.
12. Cao P, Zhang X, IPark I, Najac C, Nelson SJ, Ronen S and Larson PEZ, 1H-13C independently tuned radiofrequency surface coil applied for in vivo hyperpolarized MRI, Magn Reson Med 2016; 76: 1612–1620.
Matching
13. Mispelter, M. Lupu, and A. Briguet, NMR Probeheads: for Biophysical and Biomedical Experiments, Imperial College Press: London, 2006.
Volume Coils
14. Hayes CE, Edelstein WA, Schenck JF, Mueller OM and Eash M, An efficient, highly homogeneous radiofrequency coil for whole-body NMR imaging at 1.5 T. J Magn Reson 1985; 63: 622-628.
15. Watkins JC, Fukushima E, High-pass bird-cage coil for nuclear- magnetic resonance, Rev Sci Instr1988; 59(6): 926-929.
16. Joseph PM, Lu D, A technique for double resonant operation of birdcage imaging coils, IEEE Transactions Med. Imag 1989; 8 (3): 286-294.
17. Rath AR, Design and performance of a double-tuned bird-cage coil, J Magn Reson 1990; 86: 488-495.
18. Isaac G, Schnall MD, Lenkinski RE and Vogele K, A design for a double-tuned birdcage coil for use in an integrated MRI/MRS examination. J Magn Reson, 89, 41-50, 1990.
19. Fitzsimmons JR, Beck B, Brooker HR, Double resonant quadrature birdcage. Magn Reson Med 1993; 30: 107-114.
20. Murphy-Boesch J, Srinivasan R, Carvajal L and Brown TR, Two Configurations of the Four-Ring Birdcage Coil for 1H Imaging and 1H-Decoupled 31P Spectroscopy of the Human Head. J. Magn. Res.B 1994; 103: 103-114.
21. Vaughan JT, Hetherington HP, Out JO, Pan JW, Pohost GM, High frequency volume coils for clinical NMR imaging and spectroscopy. Magn Res Med 1994;32: 206-218.
22. Amari S, Ulu AM, Bornemann J, Van Zijl PCM, Barker PB, Multiple tuning of birdcage resonators. Magn Reson Med 1997; 37: 243-251.
23. Shen GX, Boada FE, Thulborn KR, Dual-frequency, dual-quadrature, birdcage RF coil design with identical b1 pattern for sodium and proton imaging of the human brain at 1.5 T. Magn Reson Med 1997; 38: 717-725.
24. Shen GX, Boada FE, Thulborn KR, Experimentally verified, theoretical design of dual-tuned, low-pass birdcage radiofrequency resonators for magnetic resonance imaging and magnetic resonance spectroscopy of human brain at 3.0 Tesla, Magn Reson Med 1999; 41: 268-275.
25. Matson GB, Vermathen P, Hill TC, A practical double-tuned 1H/31P quadrature birdcage headcoil optimized for 31P operation, Magn Res Med 1999; 42: 173-182.
26. Hudson AMJ, Köckenberger W and Bowtell RW, Dual resonant birdcage coils for 1H detected 13C microscopic imaging at 11.7 T, MAGMA 2000; 10: 61-68.
27. Tomanek B, Volotovskyy V, Gruwel MLH, McKenzie E, King SB, Double-frequency birdcage volume coils for 4.7T and 7T, Conc. Magn Reson B: Magn Reson Eng 2005; 26B(1): 16-22.
28. Duan Y, Peterson BS, Liu F, Brown TR, Ibrahim TS, Kangarlu A, Computational and experimental optimization of a double-tuned 1H/31P four-ring birdcage head coil for MRS at 3T, J Magn Reson Imag 2009; 29: 13-22.
29. Yahya A, De Zanche N and Allen PS, A dual-tuned transceive resonator for 13C{1H} MRS: two open coils in one, NMR in Biomed 2013; 26: 533–541.
30. Pratt R, Giaquinto R, Ireland C, Daniels B, Loew W, Higano N, Cao X, Thomen R, Woods J and Dumoulin CL, A novel switched frequency 3He/1H high-pass birdcage coil for imaging at 1.5 tesla, Conc. Magn Reson B: Magn Reson Eng 2015; 45B(4): 174–182. Phased Arrays
31. Roemer PB, Edelstein WA, Hayes CE, Souza SP, Mueller OM, The NMR phased array, Magn Res Med 1990; 16: 192-225.
32. Avdievich NI, Hetherington HP. 4 T Actively detuneable double-tuned 1H/31P head volume coil and four-channel 31P phased array for human brain spectroscopy. J Magn Reson 2007;186(2):341-346.
33. Ha S, Hamamura MJ, Nalcioglu O and Muftuler LT, A PIN diode controlled dual-tuned MRI RF coil and phased array for multi nuclear imaging, Phys. Med. Biol 2010; 55: 2589–2600.
34. Avdievich NI, Transceiver Phased Arrays for Human Brain Studies at 7T, Appl Magn Reson 2011; 41(2): 483-506.
35. Kim J-H, Moon CH, Park B-W, Furlan A, Zhao T, Bae KT, Multichannel transceiver dual-tuned RF coil for proton/sodium MR imaging of knee cartilage at 3 T, Magn Reson Imag 2012; 30: 562-571.
36. Qian Y, Zhao T, Wiggins GC, Wald LL, Zheng H, Weimer J and Boada FE, Sodium imaging of human brain at 7 T with 15-channel array coil, Magn Res Med 2012; 68: 1808–1814.
37. Brown R, Madelin G, Lattanzi R, Chang G, Regatte RR, Sodickson DK and Wiggins GC, Design of a nested eight-channel sodium and four-channel proton coil for 7T knee imaging, Magn Res Med 2013; 70: 259-268.
38. Van der Velden TA, Italiaander M, van der Kemp WJM, Raaijmakers AJE, Schmitz AMT, Luijten PR, Boer VO and Klomp DWJ, Radiofrequency configuration to facilitate bilateral breast 31P MR spectroscopic imaging and high-resolution MRI at 7 Tesla, Magn Res Med 2015; 74: 1803–1810.
39. Shajan G, Mirkes C, Buckenmaier K, Hoffmann J, Pohmann R and Scheffler K, Three-layered radio frequency coil arrangement for sodium MRI of the human brain at 9.4 Tesla, Magn Res Med 2016; 75: 906–916.
40. Mirkes C, Shajan S, Bause J, Buckenmaier K, Hoffmann J and Scheffler K, Triple-quantum-filtered sodium imaging at 9.4 Tesla , Magn Res Med 2016; 75: 1278–1289.
41. Brown R, Lakshmanan K, Madelin G, Alon L, Chang G, Sodickson DK, Regatte RR and Wiggins GC, A flexible nested sodium and proton coil array with wideband matching for knee cartilage MRI at 3T, Magn Res Med 2016; 76: 1325–1334.
42. Brown R, Lakshmanan K, Madelin G, Parasoglou P. A nested phosphorus and proton coil array for brain magnetic resonance imaging and spectroscopy. Neuroimage 2016;124(Pt A):602-611.
Safety procedure
43. Hoffmann J, Henning A, Giapitzakis IA, Scheffler K, Shajan G, Pohmann R and Avdievich NI (April-2015) Safety testing and operational procedures for self-developed radiofrequency coils NMR in Biomed 2016; 29(9): 1131–1144.