Molecular fMRI: Imaging Probes for Brain Functions & Circuits
Synopsis
A new experimental approach termed “molecular fMRI” aims to provide direct, minimally-invasive measures of neural function based on the application of molecular probes detectable in time-resolved MRI experiments. In this talk, we discuss the design and application of suitable probes for molecular fMRI, including their initial deployment for imaging several types of signaling molecules in the living brain. By improving the technology with more sensitive contrast agents and better brain delivery strategies, it will be possible to measure and map an expanding array of neurophysiological processes in animals and ultimately in humans.
Highlights
• MRI contrast agents can be designed to detect molecular-level hallmarks of neural activity.
• Molecular fMRI using intracranially-injected sensors enables detection and mapping of specific physiological processes in animal brains.
• New high sensitivity approaches may enable truly noninvasive experiments and translation of molecular fMRI strategies to human subjects
Target audience
This talk will be of interest to magnetic resonance experts interested in innovative chemically-based approaches to monitoring neurophysiological variables, as well as to neuroscientists concerned with mesoscale dynamic mapping of molecular and cellular-level processes in the brain.Outcome/Objectives
Following this lecture, learners will be familiar with the experimental approach of molecular fMRI; they will be able to describe the rationale for this methodology, some of its key achievements to date, and some of the challenges facing its future development.Purpose
Functional brain imaging with fMRI has transformed neuroscience and is increasingly being recognized as a powerful tool for analysis of neural systems in animals. Standard fMRI methods are based on the detection of hemodynamic responses elicited by neural activity. Because hemodynamic effects are only indirectly related to underlying neuronal events, fMRI typically lacks specificity for individual molecular and cellular components of brain function. The new experimental approach of “molecular fMRI” aims to circumvent these limitations by combining MRI readouts with the MRI-detectable chemical probes that are directly sensitive to neuronal processes such as neurotransmitter release, intracellular signaling, and gene expression. Using such probes it should be possible to measure and map molecular and cellular components of neural activity over large regions of the brain, initially in animals and ultimately in human subjects.Methodology
Most MRI-detectable imaging agents provide lower sensitivity to molecular events than probes used in optical or nuclear imaging. Unlike optical probes, MRI agents can be detected in opaque specimens however, and unlike nuclear imaging probes, their potency can be modulated by biochemical events. These two advantages give MRI probes special capability for monitoring physiology in optically obscure structures like mammalian brains. Most MRI contrast agents work by T1 or T2-dependent mechanisms, and neural activity sensors can be created by linking changes in the T1 or T2 potency of such agents to a target molecule of interest. Designing appropriate sensors is difficult, but several approaches have proved fruitful so far. By delivering the resulting probes to sites of action in the brain, specific components of neural function can be studied.Results & Discussion
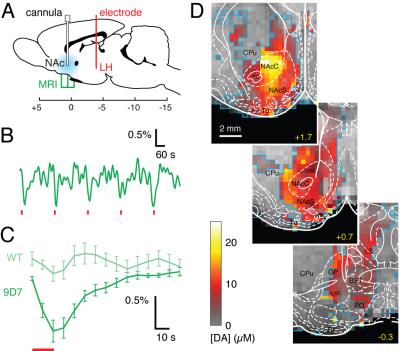
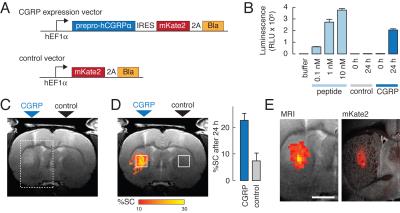
We consider three classes of MRI probes that have been successfully deployed by our laboratory in animal brains. A first class comprises T1 agents formed from paramagnetic metalloproteins engineered to sense monoamine neurotransmitters. These probes have been used to produce the first maps of dopamine release dynamics in live rat brains (Figure 1), and were recently used also to map serotonin reuptake and the action of pharmacological agents that perturb neurotransmitter reuptake. The second class of probes comprises nanoparticle-based sensors designed to detect calcium ion fluctuations via changes in T2-weighted MRI. A calcium probe tuned for monitoring extracellular calcium concentrations is able to report the effects of multiple neuronal stimuli in vivo, and may be the most generalizable molecular fMRI tool developed to date. A third class of probes is based on the novel concept of “hijacking” endogenous hemodynamics to produce dynamic MRI contrast dependent on specific molecular targets. Such probes can be detected at concentrations more than a 1,000 times lower than conventional T1 contrast agents, and may offer unique advantages as exogenous or genetically-encoded reporters (Figure 2).Conclusion
Results presented here demonstrate the possibility of developing and applying imaging probes for molecular fMRI in multiple forms. Studies performed so far reveal novel aspects of neurotransmitter transport in the brain and establish platforms for expanding efforts to map brain functions and circuitry at mesoscale using MRI-based approaches. Remaining hurdles include the need for improvements in brain delivery, probe sensitivity, and access to additional parameters of brain physiology. As molecular fMRI technology develops however, we expect it to address an increasing array of problems in preclinical and eventually clinical neuroscience research.Acknowledgements
I thank my lab members who performed this work, particularly Taekwan Lee, Nan Li, Aviad Hai, Satoshi Okada, and Ben Bartelle. I also acknowledge collaborators who contributed to our results, as well as funding from the NIH (including the NIH BRAIN Initiative), DARPA, the Parkinson's Disease Foundation, and the MIT Simons Center.References
1. Bartelle, B. B., Barandov, A., & Jasanoff, A. (2016) “Molecular fMRI,” J. Neurosci. 36: 4139-48.
2. Lee, T., Cai, L. X., Lelyveld, V. S., Hai, A., & Jasanoff, A. (2014) “Molecular-level functional magnetic resonance imaging of dopaminergic signaling,” Science 344: 533-5.
3. Hai, A., Cai, L. X., Lee, T., Lelyveld, V. S., & Jasanoff, A. (2016) “Molecular fMRI of serotonin transport,” Neuron 92: 754-65.
4. Rodriguez, E., Lelyveld, V. S., Atanasijevic, T., Okada, S., & Jasanoff, A. (2014) “Magnetic nanosensors optimized for rapid and reversible self-assembly,” Chem. Commun. 50: 3595-8.
5. Desai, M., Slusarczyk, A. L., Chapin, A., Barch, M., & Jasanoff, A. (2016) “Molecular imaging with engineered physiology,” Nat. Commun. 7: 13607.
Figures