Imaging Techniques: Current & Future
Synopsis
Atherosclerosis, a systemic disease affecting large and medium sized arterial vessel walls is a leading cause of mortality in the world. MRI is quickly becoming the imaging modality of choice for visualizing atherosclerosis in the vessel wall. Atherosclerosis is evaluated in vivo by multi-contrast dark blood turbo spin echo imaging to evaluate plaque burden and composition. DCE- MRI can be used to evaluate plaque permeability. Recently, quantitative MR imaging in the form of T1 and T2 mapping of the vessel wall and on evaluating 4D flow, shear stress and circumferential strain in the arterial tree have become popular.
Introduction:
Atherosclerosis due to its sequelae and thrombotic complications is amongst the leading causes of mortality and morbidity in the world 1. It is a systemic arterial disease affecting large and medium sized arterial vessel walls 2. Atherosclerotic lesions are heterogeneous and can consist of connective tissue extracellular matrix; cholesterol and its esters; cells such as monocyte-derived macrophages, T-lymphocytes, and smooth muscle cells, and/or thrombotic material 3,4. Magnetic resonance imaging is quickly becoming the imaging modality of choice for visualizing atherosclerosis in the vessel wall (Figure 1) 5-9. While luminal obstruction can be visualized by MR angiography, evaluating atherosclerotic plaques in vivo requires visualization of the vessel wall itself 10.2D dark blood MRI:
The most commonly used method for visualizing the arterial vessel wall is 2D double inversion recovery (DIR) with varying contrasts 11. This technique provides suppression of signal from flowing blood and good visualization of atherosclerotic lesions. Multi contrast dark blood MRI can differentiate plaque components by biophysical and biochemical parameters such as chemical composition, water content, physical state, molecular motion, or diffusion 12-16. Typically, a time of flight MRA is used in conjunction with dark blood T1, T2 and proton density weighted spin echo images to accomplish this task 15,17-20. Commonly assessed endpoints using this approach include vessel wall morphometric (wall area, wall thickness, normalized wall index etc.) and plaque composition (lipid rich necrotic core, calcification, intra plaque hemorrhage etc.) 21-23.3D dark blood MRI:
2D DIR imaging tends to be slow and time consuming and to improve speed of acquisition, several strategies to improve speed of acquisition have been previously adopted. Of late, the emphasis has also been to switch to 3D acquisitions using sampling perfection with application optimized contrasts using different flip angle evolution (Figure 2) (SPACE, aka, CUBE, VISTA) 24,25. Additional methods to obtain dark blood contrast include the use of diffusion preparatory pulses or using motion sensitized driven equilibrium (MSDE) preparation 26-30. Additionally, Simultaneous non-contrast Angiography and intra Plaque hemorrhage (SNAP) acquisitions (Figure 3) can also be used for 3D imaging 31. These combined sequences can fulfill the multi contrast requirements for plaque composition assessments using isotropic 3D acquisitions that allow multi-planar reformatting of the images.Dynamic contrast enhanced (DCE) MRI:
DCE-MRI consists in the rapid, serial acquisition of T1-weighted MR images of a volume of interest while a T1 shortening, gadolinium (Gd) based, contrast agent is injected 32-37. During imaging, the contrast agent extravasates from the plasma compartment, and causes MR signal enhancement in permeable tissues (such vulnerable atherosclerotic plaques). From the kinetics of signal enhancement, several tissues’ properties, such as microvascular volume and permeability, can be measured. Initial approaches to DCE-MRI were 2D in nature due to the stringent temporal resolution requirements but lately, 3D approaches have also been implemented 26,36. Both kinetic modeling (k-trans, Vp etc.) and non-model approaches (AUC, time-to-peak, uptake slope etc.) can be used to evaluate DCE-MRI data.Quantitative vessel wall MR imaging:
T1, T2 and proton density-weighted images are relatively qualitative and reproducibility is affected by several factors, such as coil positioning, sequence parameters and observer variability. Recently, quantitative T1 and T2 mapping approaches have been used to quantitatively evaluate the vessel wall with high reproducibility and can potentially be beneficial for longitudinal imaging and for evaluating data obtained in multi center settings 38. A robust method for this approach is by using a 3D gradient echo sequence with time efficient motion-sensitized preparation for blood suppression together with variable flip angle and variable TE preparation times for combined T1 and T2 estimation 38 (Figure 4).Phase contrast and 4D flow:
2D phase contrast cine has been used for evaluating through plane flow for decades. To facilitate a comprehensive assessment of flow in an entire three-dimensional volume, three-dimensional CINE phase contrast (3D CINE PC-MRI or 4D flow MRI) has been developed 39-41. From this data, vessel wall shear stresses (WSS) can be computed with relative ease 42. WSS has been shown to relate with pathophysiology and areas of low shear are associated with more plaque buildup. Other relevant biomarkers for atherosclerosis that can be obtained from 4D flow MRI include viscous energy loss, and pressure differences 43,44.Circumferential strain:
Changes of arterial wall strain pattern may be associated with strokes and other complications of atherosclerosis. Displacement encoding with stimulated echoes (DENSE) sequence can be used to image the motion of the common carotid artery wall and map the two-dimensional (2D) circumferential strain 45,46.Future progress:
Vessel wall imaging is reasonably established at 1.5T and 3T. Future advances will include implementation of the approaches popularized at lower field strengths to high fields such as 7T and beyond 47,48. Further developments in acceleration to yield faster imaging are also needed to make this routinely used in the clinic. Some potential approaches will include the use of compressed sensing 49,50 or other acceleration techniques, for example Hyperecho-PROPELLOR 51. Finally, multimodality imaging is becoming more commonplace with the advent of PET-MRI scanners. Vessel wall imaging on a PET/MRI scanner may provide more valuable data regarding the entire atherosclerotic cascade by providing complementary information of various facets of atherosclerosis in the same imaging session 52,53.Acknowledgements
No acknowledgement found.References
1 Libby, P. Atherosclerosis: the new view. Sci Am 286, 46-55 (2002).
2 Libby, P. Inflammation in atherosclerosis. Nature 420, 868-874, doi:10.1038/nature01323 (2002).
3 Davies, M. J. Stability and instability: two faces of coronary atherosclerosis. The Paul Dudley White Lecture 1995. Circulation 94, 2013-2020 (1996).
4 Stary, H. C. et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 92, 1355-1374 (1995).
5 Phinikaridou, A. et al. Molecular MRI of atherosclerosis. Molecules 18, 14042-14069, doi:10.3390/molecules181114042 (2013).
6 Kerwin, W. S., Hatsukami, T., Yuan, C. & Zhao, X. Q. MRI of carotid atherosclerosis. AJR Am J Roentgenol 200, W304-313, doi:10.2214/AJR.12.8665 (2013).
7 Underhill, H. R., Hatsukami, T. S., Fayad, Z. A., Fuster, V. & Yuan, C. MRI of carotid atherosclerosis: clinical implications and future directions. Nat Rev Cardiol 7, 165-173, doi:10.1038/nrcardio.2009.246 (2010).
8 Yuan, C., Oikawa, M., Miller, Z. & Hatsukami, T. MRI of carotid atherosclerosis. J Nucl Cardiol 15, 266-275, doi:10.1016/j.nuclcard.2008.02.001 (2008).
9 Weinreb, D. B., Aguinaldo, J. G., Feig, J. E., Fisher, E. A. & Fayad, Z. A. Non-invasive MRI of mouse models of atherosclerosis. NMR Biomed 20, 256-264, doi:10.1002/nbm.1148 (2007).
10 Abolmaali, N. et al. Vessel wall MRI of the thoracic aorta: correlation to histology and transesophageal ultrasound. Preliminary results. Rofo 174, 568-572, doi:10.1055/s-2002-28272 (2002).
11 El Aidi, H. et al. Cross-sectional, prospective study of MRI reproducibility in the assessment of plaque burden of the carotid arteries and aorta. Nat Clin Pract Cardiovasc Med 6, 219-228, doi:10.1038/ncpcardio1444 (2009).
12 Dai, Y. et al. Comparison study between multicontrast atherosclerosis characterization (MATCH) and conventional multicontrast MRI of carotid plaque with histology validation. J Magn Reson Imaging 45, 764-770, doi:10.1002/jmri.25444 (2017).
13 Liu, W. et al. Segmentation of carotid plaque using multicontrast 3D gradient echo MRI. J Magn Reson Imaging 35, 812-819, doi:10.1002/jmri.22886 (2012).
14 Li, F. et al. Scan-rescan reproducibility of carotid atherosclerotic plaque morphology and tissue composition measurements using multicontrast MRI at 3T. J Magn Reson Imaging 31, 168-176, doi:10.1002/jmri.22014 (2010).
15 Yarnykh, V. L. et al. Multicontrast black-blood MRI of carotid arteries: comparison between 1.5 and 3 tesla magnetic field strengths. J Magn Reson Imaging 23, 691-698, doi:10.1002/jmri.20562 (2006).
16 Clarke, S. E., Hammond, R. R., Mitchell, J. R. & Rutt, B. K. Quantitative assessment of carotid plaque composition using multicontrast MRI and registered histology. Magn Reson Med 50, 1199-1208, doi:10.1002/mrm.10618 (2003).
17 Itskovich, V. V. et al. Quantification of human atherosclerotic plaques using spatially enhanced cluster analysis of multicontrast-weighted magnetic resonance images. Magn Reson Med 52, 515-523, doi:10.1002/mrm.20154 (2004).
18 Saam, T. et al. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol 25, 234-239, doi:10.1161/01.ATV.0000149867.61851.31 (2005).
19 Chu, B. et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 35, 1079-1084, doi:10.1161/01.STR.0000125856.25309.86 (2004).
20 Yuan, C., Miller, Z. E., Cai, J. & Hatsukami, T. Carotid atherosclerotic wall imaging by MRI. Neuroimaging Clin N Am 12, 391-401, vi (2002).
21 Mani, V. et al. Predictors of change in carotid atherosclerotic plaque inflammation and burden as measured by 18-FDG-PET and MRI, respectively, in the dal-PLAQUE study. Int J Cardiovasc Imaging 30, 571-582, doi:10.1007/s10554-014-0370-7 (2014).
22 Fayad, Z. A. et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet 378, 1547-1559, doi:10.1016/S0140-6736(11)61383-4 (2011).
23 Fayad, Z. A. et al. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J 162, 214-221 e212, doi:10.1016/j.ahj.2011.05.006 (2011).
24 Wong, S. K. et al. Atherosclerosis imaging using 3D black blood TSE SPACE vs 2D TSE. World journal of radiology 6, 192-202, doi:10.4329/wjr.v6.i5.192 (2014).
25 Mihai, G. et al. Assessment of carotid stenosis using three-dimensional T2-weighted dark blood imaging: Initial experience. J Magn Reson Imaging 35, 449-455, doi:10.1002/jmri.22839 (2012).
26 Calcagno, C. et al. Three-dimensional dynamic contrast-enhanced MRI for the accurate, extensive quantification of microvascular permeability in atherosclerotic plaques. NMR Biomed 28, 1304-1314, doi:10.1002/nbm.3369 (2015).
27 Chi, J. et al. Assessment of femoral artery atherosclerosis at the adductor canal using 3D black-blood MRI. Clin Radiol 68, e213-221, doi:10.1016/j.crad.2012.12.002 (2013).
28 Dong, L. et al. Efficient flow suppressed MRI improves interscan reproducibility of carotid atherosclerosis plaque burden measurements. J Magn Reson Imaging 32, 452-458, doi:10.1002/jmri.22274 (2010).
29 Wang, J. et al. Improved suppression of plaque-mimicking artifacts in black-blood carotid atherosclerosis imaging using a multislice motion-sensitized driven-equilibrium (MSDE) turbo spin-echo (TSE) sequence. Magn Reson Med 58, 973-981, doi:10.1002/mrm.21385 (2007).
30 Sirol, M. et al. Lipid-rich atherosclerotic plaques detected by gadofluorine-enhanced in vivo magnetic resonance imaging. Circulation 109, 2890-2896, doi:10.1161/01.CIR.0000129310.17277.E7 (2004).
31 Zhou, Z. et al. Evaluation of 3D multi-contrast joint intra- and extracranial vessel wall cardiovascular magnetic resonance. J Cardiovasc Magn Reson 17, 41, doi:10.1186/s12968-015-0143-z (2015).
32 Truijman, M. T. et al. Combined 18F-FDG PET-CT and DCE-MRI to assess inflammation and microvascularization in atherosclerotic plaques. Stroke 44, 3568-3570, doi:10.1161/STROKEAHA.113.003140 (2013).
33 Calcagno, C. et al. Gadolinium-Based Contrast Agents for Vessel Wall Magnetic Resonance Imaging (MRI) of Atherosclerosis. Curr Cardiovasc Imaging Rep 6, 11-24, doi:10.1007/s12410-012-9177-x (2013).
34 Mani, V. et al. Relationship between particulate matter exposure and atherogenic profile in "Ground Zero" workers as shown by dynamic contrast enhanced MR imaging. Int J Cardiovasc Imaging 29, 827-833, doi:10.1007/s10554-012-0154-x (2013).
35 Calcagno, C., Mani, V., Ramachandran, S. & Fayad, Z. A. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis 13, 87-99, doi:10.1007/s10456-010-9172-2 (2010).
36 Calcagno, C. et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arterioscler Thromb Vasc Biol 28, 1311-1317, doi:10.1161/ATVBAHA.108.166173 (2008).
37 Kerwin, W. S., Oikawa, M., Yuan, C., Jarvik, G. P. & Hatsukami, T. S. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med 59, 507-514, doi:10.1002/mrm.21532 (2008).
38 Coolen, B. F. et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magn Reson Med 75, 1008-1017, doi:10.1002/mrm.25634 (2016).
39 Harloff, A. et al. Comparison of blood flow velocity quantification by 4D flow MR imaging with ultrasound at the carotid bifurcation. AJNR Am J Neuroradiol 34, 1407-1413, doi:10.3174/ajnr.A3419 (2013).
40 Markl, M., Frydrychowicz, A., Kozerke, S., Hope, M. & Wieben, O. 4D flow MRI. J Magn Reson Imaging 36, 1015-1036, doi:10.1002/jmri.23632 (2012).
41 Harloff, A. et al. 3D blood flow characteristics in the carotid artery bifurcation assessed by flow-sensitive 4D MRI at 3T. Magn Reson Med 61, 65-74, doi:10.1002/mrm.21774 (2009).
42 Harloff, A. et al. Wall shear stress distribution at the carotid bifurcation: influence of eversion carotid endarterectomy. Eur Radiol 23, 3361-3369, doi:10.1007/s00330-013-2953-4 (2013).
43 Casas, B., Lantz, J., Dyverfeldt, P. & Ebbers, T. 4D Flow MRI-based pressure loss estimation in stenotic flows: Evaluation using numerical simulations. Magn Reson Med 75, 1808-1821, doi:10.1002/mrm.25772 (2016).
44 Barker, A. J. et al. Viscous energy loss in the presence of abnormal aortic flow. Magn Reson Med 72, 620-628, doi:10.1002/mrm.24962 (2014).
45 Nederveen, A. J., Avril, S. & Speelman, L. MRI strain imaging of the carotid artery: present limitations and future challenges. J Biomech 47, 824-833, doi:10.1016/j.jbiomech.2014.01.014 (2014).
46 Lin, A. P. et al. Circumferential strain in the wall of the common carotid artery: comparing displacement-encoded and cine MRI in volunteers. Magn Reson Med 60, 8-13, doi:10.1002/mrm.21621 (2008).
47 Lopez Gonzalez, M. R. et al. Atherosclerotic Carotid Plaque Composition: A 3T and 7T MRI-Histology Correlation Study. J Neuroimaging 26, 406-413, doi:10.1111/jon.12332 (2016).
48 Koning, W. et al. MRI of the carotid artery at 7 Tesla: quantitative comparison with 3 Tesla. J Magn Reson Imaging 41, 773-780, doi:10.1002/jmri.24601 (2015).
49 Li, B. et al. Compressed sensing based simultaneous black- and gray-blood carotid vessel wall MR imaging. Magn Reson Imaging 38, 214-223, doi:10.1016/j.mri.2017.01.013 (2017).
50 Yuan, J. et al. Three-dimensional black-blood T2 mapping with compressed sensing and data-driven parallel imaging in the carotid artery. Magn Reson Imaging 37, 62-69, doi:10.1016/j.mri.2016.11.014 (2017).
51 Yoneyama, M. et al. Hyperecho PROPELLER-MRI: Application to rapid high-resolution motion-insensitive T2 -weighted black-blood imaging of the carotid arterial vessel wall and plaque. J Magn Reson Imaging 45, 515-524, doi:10.1002/jmri.25377 (2017).
52 Robson, P. M. et al. Coronary Artery PET/MR Imaging: Feasibility, Limitations, and Solutions. JACC Cardiovasc Imaging, doi:10.1016/j.jcmg.2016.09.029 (2017).
53 Vesey, A. T., Dweck, M. R. & Fayad, Z. A. Utility of Combining PET and MR Imaging of Carotid Plaque. Neuroimaging Clin N Am 26, 55-68, doi:10.1016/j.nic.2015.09.005 (2016).
Figures
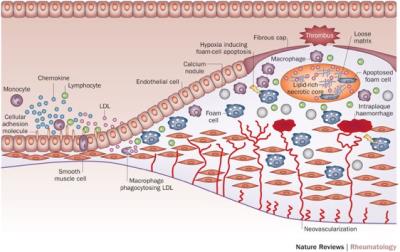
Figure 1: Pathogenesis of Atherosclerosis.
Skeoch, S. & Bruce, I. N. Nat. Rev. Rheumatol. 2015. doi:10.1038/nrrheum.2015.40

Figure 2: Sample magnetic resonance images obtained from the three vascular beds using both three-dimensional SPACE and two-dimensional turbo spin echo sequences.
Wong et al. World J Radiol. 2014 May 28; 6(5): 192–202.
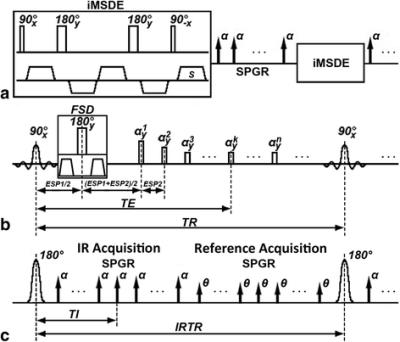
Figure 3: Sequence diagram of 3D-MERGE, T2-weighted VISTA and SNAP. (a - c) corresponds respectively to the pulse sequence diagram of 3D-MERGE, T2-weighted VISTA and SNAP. The fat saturation scheme used both for 3D-MERGE and T2-weighted VISTA is spectral presaturation inversion recovery (SPIR) and for SNAP is water selective excitation in practice but they are not shown in this schematic figure
Zhou et al. JCMR 2015 17:41DOI: 10.1186/s12968-015-0143-z
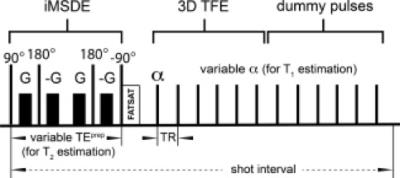
Figure 4: Carotid artery 3D black-blood T1 and T2 mapping sequence. Pulse sequence diagram for carotid artery 3D black-blood T1 and T2 mapping. Blood suppression, as well as T2-weighting, is achieved by an iMSDE prepulse. Subsequently, acquisition is performed using a segmented TFE read-out. T1 can be estimated from scans with different flip angle α, while T2 is estimated from scans with different TEprep (see also Table 1). To ensure correct T1 and T2 quantification, after each TFE shot, steady-state conditions are restored by a series of dummy pulses.
Coolen, B. F., et al Mag Res Med 2016, 75: 1008–1017. doi:10.1002/mrm.25634