Coronary, Aorta & Peripheral Vessel Wall MR Imaging
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Magnetic resonance (MR) has emerged as a leading noninvasive imaging modality for assessing the wall disease beyond revealing luminal stenosis. Continued technical innovations are being proposed for MR atherosclerosis imaging, particularly vessel wall imaging, at coronary, aorta and peripheral vascular beds. Detailed knowledge about these techniques would foster adoption of MR as an effective imaging tool in future research and clinical practice. The present lecture will focus on technical developments in MR vessel wall imaging of these arteries.
Introduction
Atherosclerosis is a systemic inflammatory disease affecting the wall of large and medium sized arterial vessel vessels such as the coronary, aorta, and peripheral arteries. Presently, the evaluation of the disease in a clinical setting is limited to the assessment of the degree of arterial luminal narrowing by imaging. However, a substantial proportion of sudden ischemic events arise from an atherosclerotic plaque that is of moderate or mild stenosis as a result of positive wall remodeling [1]. Magnetic resonance (MR) has emerged as a leading noninvasive imaging modality for assessing the wall disease beyond revealing luminal stenosis. Continued technical innovations are being proposed for MR atherosclerosis imaging, particularly vessel wall imaging, at coronary, aorta and peripheral vascular beds. Detailed knowledge about these techniques would foster adoption of MR as an effective imaging tool in future research and clinical practice. The present lecture will focus on technical developments in MR vessel wall imaging of these arteries.Coronary Vessel Wall MR Imaging
Coronary artery disease is the leading cause of death worldwide. Imaging of the coronary arteries, particularly the vessel wall, is challenged by its small luminal caliber and wall thickness and continuous movement with both the cardiac and respiratory cycles. Therefore, long-standing research interest has been focused on this disease.
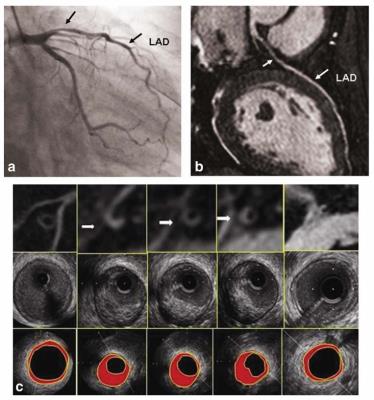
Dark-blood MR has shown great potential in coronary wall/plaque visualization and quantification. Two-dimensional (2D) dark-blood MR based on double inversion recovery (DIR) prepared turbo spin-echo (TSE) was the first development for coronary plaque imaging [2, 3] and, to date, is still most commonly used in clinical studies [4-7]. To reduce the effects of cardiac motion, data has to be acquired during the rest period within each cardiac cycle using electrocardiogram triggering. Respiratory motion artifacts are minimized with breath holding [3, 8, 9] or, more typically, using free-breathing motion adapted navigator [2]. For plaque morphologic measurements at the proximal coronary segments, this technique demonstrates high reproducibility [10] and good correlation with intravascular ultrasound (IVUS), the clinical reference method [11] (Figure 1). To accommodate fast heart rates in the heart transplant patient population, Lin et al. proposed using steady-state free precession (SSFP) instead of TSE to allow for a shorter time window in each heartbeat due to its higher efficiency in data sampling [12].
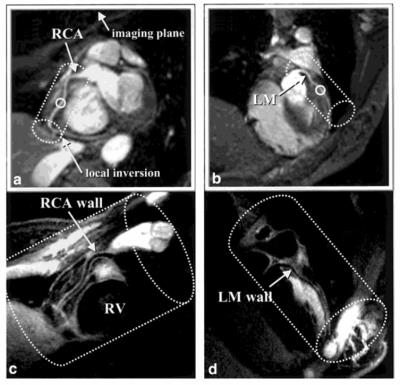
Three-dimensional (3D) dark-blood MR is currently a major focus within the technical development community. Compared to 2D acquisitions, 3D imaging allows for higher spatial resolution and larger spatial coverage per unit acquisition time. However, several challenges are associated with 3D methods, including suboptimal suppression of blood signals and long scan time. An appealing solution dates back to 2001 when Botnar et al. combined a modified DIR preparation with a spiral sampling trajectory to achieve efficient 3D imaging [13] (Figure 2). This dark-blood preparative scheme, although widely adopted by many other 3D MR techniques [14-16], requires sufficient inflow of “dark” blood due to its flow-dependent nature and thus is restricted to visualization of proximal vessel segments when using an in-plane imaging strategy. A flow-independent dark-blood method has recently been proposed based on the subtraction of data with and without T2-preparation [17, 18]. Low navigator gating efficiency due to respiratory drift or erratic breathing is a major cause for lengthy 3D coronary vessel wall imaging [19, 20]. The feasibility of eliminating navigator gating by using image navigators to achieve near 100% respiratory efficiency has recently been demonstrated by Scott et al. [15].
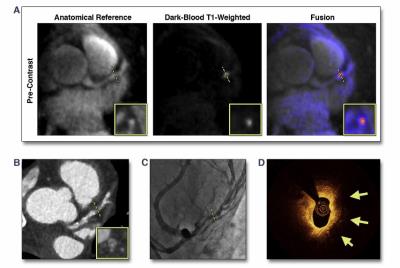
In addition to morphologic imaging of coronary plaques, another research focus lies in developing dark-blood MR techniques to discern plaque component and biological processes. These techniques can be categorized into two major classes: 1) unenhanced imaging to identify vulnerable plaque constituents such as intraplaque hemorrhage [21, 22] and calcification [16]; 2) delayed-enhanced imaging to evaluate inflammatory status [23-26]. Most of these studies utilize a conventional inversion-recovery 3D spoiled gradient echo (GRE) sequence to yield a heavy T1-weighting and background signal suppression for distinct detection of short-T1 plaque features; a separate magnetic resonance angiography is needed to provide spatial reference for anatomy. In addition, spatial resolution and/or coverage is often sacrificed to achieve a clinically tolerable scan time. More recently, Xie et al. proposed coronary atherosclerosis T1-weighted characterization with integrated anatomical reference (CATCH) method which allows for the identification of intraplaque hemorrhage and delayed plaque enhancement with whole-heart coverage and 100% respiratory efficiency (Figure 3).
Aortic Vessel Wall MR Imaging
The aorta is commonly involved in the atherosclerosis. MR imaging has been widely employed for assessing atherosclerosis in the thoracic and abdominal aorta. Compared to the coronary arteries, the aorta is easier to image with MR because of its large size and non-tortuous course.
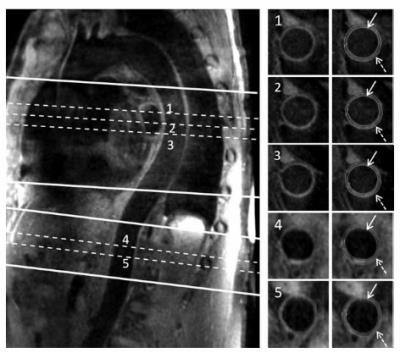
Most sequences used for dark-blood MR of the aorta are 2D TSE, which renders them susceptible to partial volume effects and compromised spatial coverage when cross-sectional imaging is prescribed [27]. Koktzoglou et al. proposed a 3D diffusion-prepared SSFP technique that allows for isotropic-resolution imaging of the entire thoracic aorta with a sagittal oblique volume [28]. Due to the motion sensitivity of the diffusion preparation, however, signal loss may be present in the aortic root that is associated with drastic cardiac and respiratory motion. To accommodate 3.0T where magnetic field is less homogeneous, other 3D MR sequences, such as DIR prepared 3D GRE (or turbo field echo) [29] and 3D TSE with variable refocusing flip angles [30], were proposed. The 3D TSE sequence is relatively more time efficient as the entire descending aorta can be covered with a sagittal oblique scan. Eikendal et al. demonstrated excellent reproducibility for quantification of descending thoracic aortic wall morphology in healthy, young adults, suggesting the potential of using this sequence for pre-clinical detection of atherosclerosis disease [31]. However, due to the lack of navigator and ECG-triggering, the signal from the ascending thoracic aorta and arch may not be satisfactory. Motion compensation has been recently integrated into the sequence [32] (Figure 4). Another 3D GRE sequence, by exploiting 2D spatially selective excitation, alleviates the need for breathing motion compensation and allows for short scan time [33].
Efforts have also been focused on developing techniques for the detection of vulnerable plaque components and inflammation activities in the aortic vessel wall. Conventional 2D multi-contrast TSE were initially used for identifying, for example, fibrous cap and lipid core [34]. To expedite 2D imaging and provide T1-independent blood signal suppression required for post-contrast imaging, Peel et al. proposed a combination of quadruple inversion recovery and reduced field-of-view and demonstrated the improvement in abdominal aortic wall imaging [35]. A 3D MR sequence, named SNAP, was recently shown to be able to detect intraplaque hemorrhage [36].
Peripheral Vessel Wall MR Imaging
Peripheral artery disease (PAD) has become a public health issue worldwide and major cause of PAD is atherosclerosis. The peripheral arteries are ideal for vessel wall imaging due to their superficial location, long length, straight course, and lack of motion, but they have not been extensively investigated.
2D TSE with dark-blood preparation was the first MR method and most widely used for peripheral vessel wall imaging. Its feasibility and reproducibility to assess plaque volume in the superficial femoral artery has been demonstrated [37]. Using the technique, Li et al. detected both eccentric lesions and concentric lesions in PAD patients and identified an association between plaque eccentricity and advanced plaque features, such as larger plaque burden, more lipid content, and increased calcification, in the superficial femoral artery [38]. However, 2D TSE is limited in clinical settings due to its poor slice resolution, long scan times, and limited spatial coverage.
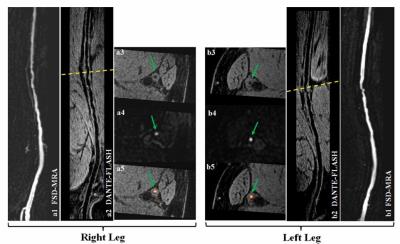
Several dark-blood 3D techniques have been developed including Motion-sensitized driven equilibrium (MSDE) prepared 3D GRE [39] and 3D TSE with variable refocusing flip angles [30, 40]. However, these two methods have inherent drawbacks. MSDE-GRE, although fast, is sensitive to the B1 field inhomogeneity because of the typically used large field of view (FOV) and large-flip-angle radiofrequency pulses in the MSDE preparation. This issue can be exacerbated at higher magnetic field strengths such as 3.0T. As a result, the quality of flow signal suppression and wall delineation may not be consistently satisfactory throughout the long arterial segment. Compared with MSDE-GRE, 3D TSE requires relatively long scan times. Recently, two GRE-based techniques were proposed to overcome the limitations above, including 3D water-selective SSFP-echo [41] and DANTE (delay alternating with nutation for tailored excitation) prepared GRE [42] (Figure 5), both of which allows for a rapid, robust imaging of the peripheral arteries at 3.0T. In addition, flow-independent vessel wall imaging techniques have been proposed to achieve reliable dark-blood effect in the presence of potentially complex flow pattern in PAD patients [43, 44]. Compositional imaging of peripheral atherosclerotic plaque has been mainly focused on calcification detection using susceptibility weighted imaging [45, 46] and dark- and gray-blood dual contrast imaging [46].
Conclusion
Over the past two decades, numerous technical advances have been introduced to MR vessel wall imaging that allow for reliable visualization and characterization of the coronary, aortic, and peripheral vessel wall. The modality has helped us better understand atherosclerosis and contribute to improvement in atherosclerosis prevention and treatment outcome. Further developments in MR vessel wall imaging techniques will strengthen its pivotal role in basic atherosclerosis research, clinical trials, and clinical practice.Acknowledgements
No acknowledgement found.References
1. Varnava, A.M., P.G. Mills, and M.J. Davies, Relationship Between Coronary Artery Remodeling and Plaque Vulnerability. Circulation, 2002. 105(8): p. 939-943.
2. Botnar, R.M., et al., Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation, 2000. 102(21): p. 2582-2587.
3. Fayad, Z.A., et al., Noninvasive in vivo human coronary artery lumen and wall imaging using black-blood magnetic resonance imaging. Circulation, 2000. 102(5): p. 506-510.
4. Kim, W.Y., et al., Subclinical coronary and aortic atherosclerosis detected by magnetic resonance imaging in type 1 diabetes with and without diabetic nephropathy. Circulation, 2007. 115(2): p. 228-235.
5. Miao, C., et al., Positive remodeling of the coronary arteries detected by magnetic resonance imaging in an asymptomatic population: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology, 2009. 53(18): p. 1708-1715.
6. Kim, W.Y., et al., Three-dimensional black-blood cardiac magnetic resonance coronary vessel wall imaging detects positive arterial remodeling in patients with nonsignificant coronary artery disease. Circulation, 2002. 106(3): p. 296-299.
7. Gerretsen, S.C., et al., Visualization of coronary wall atherosclerosis in asymptomatic subjects and patients with coronary artery disease using magnetic resonance imaging. PloS one, 2010. 5(9).
8. Koktzoglou, I., O. Simonetti, and D. Li, Coronary artery wall imaging: initial experience at 3 Tesla. Journal of magnetic resonance imaging : JMRI, 2005. 21(2): p. 128-132.
9. Macedo, R., et al., MRI detects increased coronary wall thickness in asymptomatic individuals: The multi-ethnic study of atherosclerosis (MESA). Journal of Magnetic Resonance Imaging, 2008. 28(5): p. 1108-1115.
10. Hazirolan, T., et al., Reproducibility of black-blood coronary vessel wall MR imaging. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance, 2005. 7(2): p. 409-413.
11. He, Y., et al., Accuracy of MRI to identify the coronary artery plaque: A comparative study with intravascular ultrasound. Journal of Magnetic Resonance Imaging, 2012. 35(1): p. 72-78.
12. Lin, K., et al., Black-blood steady-state free precession (SSFP) coronary wall MRI for cardiac allografts: A feasibility study. Journal of Magnetic Resonance Imaging, 2012. 35(5): p. 1210-1215.
13. Botnar, R.M., et al., 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magnetic Resonance in Medicine, 2001. 46(5): p. 848-854.
14. Priest, A.N., et al., Magnetic resonance imaging of the coronary vessel wall at 3 T using an obliquely oriented reinversion slab with adiabatic pulses. Magnetic Resonance in Medicine, 2005. 54(5): p. 1115-1122.
15. Scott, A.D., J. Keegan, and D.N. Firmin, High-resolution 3D coronary vessel wall imaging with near 100% respiratory efficiency using epicardial fat tracking: Reproducibility and comparison with standard methods. Journal of Magnetic Resonance Imaging, 2011. 33(1): p. 77-86.
16. Xie, G., et al., Three-dimensional coronary dark-blood interleaved with gray-blood (cDIG) magnetic resonance imaging at 3 tesla. Magnetic Resonance in Medicine, 2016. 75(3): p. 997-1007.
17. Andia, M.E., et al., Flow-independent 3D whole-heart vessel wall imaging using an interleaved T2-preparation acquisition. Magnetic Resonance in Medicine, 2013. 69(1): p. 150-157.
18. Cruz, G., et al., Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magnetic Resonance in Medicine, 2016.
19. Lin, K., et al., Effects of respiratory motion on coronary wall MR imaging: a quantitative study of older adults. The International Journal of Cardiovascular Imaging, 2013. 29(5): p. 1069-1076.
20. Taylor, A.M., et al., MR navigator-echo monitoring of temporal changes in diaphragm position: Implications for MR coronary angiography. Journal of Magnetic Resonance Imaging, 1997. 7(4): p. 629-636.
21. Kawasaki, T., et al., Characterization of hyperintense plaque with noncontrast T(1)-weighted cardiac magnetic resonance coronary plaque imaging: comparison with multislice computed tomography and intravascular ultrasound. JACC. Cardiovascular imaging, 2009. 2(6): p. 720-728.
22. Noguchi, T., et al., High-Intensity Signals in Coronary Plaques on Noncontrast T1-Weighted Magnetic Resonance Imaging as a Novel Determinant of Coronary Events. Journal of the American College of Cardiology, 2014. 63(10): p. 989-999.
23. Maintz, D., et al., Selective coronary artery plaque visualization and differentiation by contrast-enhanced inversion prepared MRI. European heart journal, 2006. 27(14): p. 1732-1736.
24. Yeon, S.B., et al., Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. Journal of the American College of Cardiology, 2007. 50(5): p. 441-447.
25. Ibrahim, T., et al., Serial contrast-enhanced cardiac magnetic resonance imaging demonstrates regression of hyperenhancement within the coronary artery wall in patients after acute myocardial infarction. JACC. Cardiovascular imaging, 2009. 2(5): p. 580-588.
26. Varma, N., et al., Coronary Vessel Wall Contrast Enhancement Imaging as a Potential Direct Marker of Coronary Involvement Integration of Findings From CAD and SLE Patients. JACC: Cardiovascular Imaging, 2014. 7(8): p. 762-770.
27. Fayad, Z.A., et al., In vivo magnetic resonance evaluation of atherosclerotic plaques in the human thoracic aorta: a comparison with transesophageal echocardiography. Circulation, 2000. 101(21): p. 2503-2509.
28. Koktzoglou, I., et al., Dark-Blood MRI of the Thoracic Aorta with 3D Diffusion-Prepared Steady-State Free Precession: Initial Clinical Evaluation. American Journal of Roentgenology, 2007. 189(4): p. 966-972.
29. Roes, S.D., et al., Aortic vessel wall magnetic resonance imaging at 3.0 Tesla: A reproducibility study of respiratory navigator gated free-breathing 3D black blood magnetic resonance imaging. Magnetic Resonance in Medicine, 2009. 61(1): p. 35-44.
30. Mihai, G., et al., T1-weighted–SPACE dark blood whole body magnetic resonance angiography (DB-WBMRA): Initial experience. Journal of Magnetic Resonance Imaging, 2010. 31(2): p. 502-509.
31. Eikendal, A.L.M., et al., 3D black blood VISTA vessel wall cardiovascular magnetic resonance of the thoracic aorta wall in young, healthy adults: reproducibility and implications for efficacy trial sample sizes: a cross-sectional study. Journal of Cardiovascular Magnetic Resonance, 2016. 18(1): p. 20.
32. Mihai, G., et al., Reproducibility of thoracic and abdominal aortic wall measurements with three-dimensional, variable flip angle (SPACE) MRI. Journal of magnetic resonance imaging : JMRI, 2015. 41(1): p. 202-212.
33. Mooiweer, R., et al., Fast 3D isotropic imaging of the aortic vessel wall by application of 2D spatially selective excitation and a new way of inversion recovery for black blood imaging. Magnetic resonance in medicine, 2016. 75(2): p. 547-555.
34. Kramer, C.M., et al., Magnetic resonance imaging identifies the fibrous cap in atherosclerotic abdominal aortic aneurysm. Circulation, 2004. 109(8): p. 1016-1021.
35. Peel, S.A., et al., Accelerated aortic imaging using small field of view imaging and electrocardiogram-triggered quadruple inversion recovery magnetization preparation. Journal of magnetic resonance imaging : JMRI, 2011. 34(5): p. 1176-1183.
36. Zhou, C., et al., Characterization of atherosclerotic disease in thoracic aorta: A 3D, multicontrast vessel wall imaging study. European Journal of Radiology, 2016. 85(11): p. 2030-2035.
37. Isbell, D.C., et al., Reproducibility and reliability of atherosclerotic plaque volume measurements in peripheral arterial disease with cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance, 2007. 9(1): p. 71-76.
38. Li, F., et al., The association of lesion eccentricity with plaque morphology and components in the superficial femoral artery: a high-spatial-resolution, multi-contrast weighted CMR study. Journal of Cardiovascular Magnetic Resonance, 2010. 12(1): p. 1-8.
39. Chiu, B., et al., Fast plaque burden assessment of the femoral artery using 3D black-blood MRI and automated segmentation. Medical physics, 2011. 38(10): p. 5370-5384.
40. Zhang, Z., et al., Three-dimensional T2-weighted MRI of the human femoral arterial vessel wall at 3.0 Tesla. Investigative radiology, 2009. 44(9): p. 619-626.
41. Langham, M.C., et al., Vessel-wall imaging and quantification of flow-mediated dilation using water-selective 3D SSFP-echo. Journal of Cardiovascular Magnetic Resonance, 2013. 15(1): p. 1-9.
42. Xie, G., et al., DANTE-prepared three-dimensional FLASH: A fast isotropic-resolution MR approach to morphological evaluation of the peripheral arterial wall at 3 tesla. Journal of Magnetic Resonance Imaging, 2016. 43(2): p. 343-351.
43. Brown, R., et al., Effect of blood flow on double inversion recovery vessel wall MRI of the peripheral arteries: Quantitation with T2 mapping and comparison with flow-insensitive T2-prepared inversion recovery imaging. Magnetic Resonance in Medicine, 2010. 63(3): p. 736-744.
44. Xie, J., et al., 3D flow-independent peripheral vessel wall imaging using T2-prepared phase-sensitive inversion-recovery steady-state free precession. Journal of Magnetic Resonance Imaging, 2010. 32(2): p. 399-408.
45. Yang, Q., et al., Imaging the vessel wall in major peripheral arteries using susceptibility-weighted imaging. Journal of Magnetic Resonance Imaging, 2009. 30(2): p. 357-365.
46. Langham, M.C., et al., Rapid High-resolution, Self-registered, Dual Lumen-contrast MRI Method for Vessel-wall Assessment in Peripheral Artery Disease:: A Preliminary Investigation. Academic radiology, 2016. 23(4): p. 457-467.
Figures