Hyperpolarized MRI & MRS Tracers
Synopsis
MRI relies on detecting signals in the radiofrequency range that are related to very small energy transitions of the detected molecules. While this is a blessing with regard to the harmless character of MRI, it imposes a serious problem in terms of the low sensitivity caused by almost vanishing spin polarization at ambient temperature. Increasing the sensitivity through special preparation of the spin system prior to the encoding and detection is therefore a powerful approach. The achieved hyperpolarization has enabled various applications for molecular and cellular imaging. This tutorial will summarize aspects of polarization methods, probe design and signal encoding.
Introduction and motivation
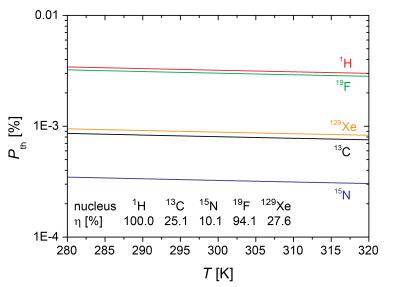
MRI suffers from intrinsic low sensitivity. The origin of this problem is an almost equal population of the spin energy levels and therefore a nearly vanishing spin polarization (see Fig. 1). The abundant pool of tissue water is therefore used in conventional imaging but it limits the ability to encode information from dilute metabolic markers relevant to molecular and metabolic imaging. Diagnostic imaging on a molecular level requires a significantly improved sensitivity. Special preparation of the spin system prior to the encoding and detection can increase the detectable magnetization significantly and is a continuing endeavour in the field of NMR and MRI. The achieved condition is called hyperpolarization. Though being of transient nature, it is a powerful approach that has enabled various novel diagnostic applications. Drug development and therapeutic monitoring will benefit significantly from specific contrast agents or metabolites with enhanced sensitivity.1,2Available techniques
Hyperpolarization is achievable through an ex situ manipulation of the magnetization beyond thermal equilibrium. Such hyperpolarized systems can be generated for various molecules of biomedical interest and the enhanced net magnetization does not influence the pharmacokinetic and pharmacological behavior. Several different nuclei have been studied in this context, including carbon and nitrogen but also some noble gases.
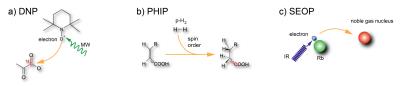
The common concept relies on employing interactions on the atomic level to achieve a highly-populated spin state. Certain spin systems can be manipulated more easily to function as a polarization precursor. Then, through various interactions, the polarization can be transferred onto the eventually detected nuclei. This enhances the net polarization from thermal conditions, P = 10–5–10–6, all the way to the theoretical limit of P = 1. Three main approaches including their basic principles (depicted in Fig. 2) will be discussed with their advantages and limitations.3 For Dynamic Nuclear Polarization (DNP), unpaired electrons are used as the initial source of magnetization which is always much higher than that of 1H protons or any other nuclei. If the electron couples with nearby nuclei through scalar or dipolar interactions, by irradiating at or around the electron Larmor frequency, the electron polarization can be transferred to polarize NMR-active nuclei. The upper limit of the polarization of the nuclei is determined by the thermal polarization of the electrons.
Techniques based on chemical interaction with para-hydrogen (PHIP and SABRE) rely on the fact that hydrogen exists in two different isomers, para- and orthohydrogen. While para-hydrogen cannot be detected directly in an NMR experiment, its spin order, however, can be converted into detectable magnetization. It is easy to increase the population of para-hydrogen from (the high-temperature limit of) 25% to 90% just by cooling to < 50 K. To create a detectable NMR signal, the two hydrogen atoms must be introduced into a system with to magnetically inequivalent sites on the target molecule for breaking their symmetry whilst remaining coupled. Different methods are used to transfer the spin order generated by PHIP into longitudinal magnetization of 13C or 15N of various tracers.
The intrinsic polarization of photons is used to generate hyperpolarized noble gases through Spin Exchange Optical Pumping (SEOP). It found early applications in lung imaging using 3He and 129Xe. In particular, Xe is a promising candidate for various applications beyond gas phase imaging, including molecular sensing. The initial step for SEOP is the production of circularly polarized light, which is then used to pump a specific electron transition in an alkali metal vapor. These electrons can later undergo spin–spin interactions with gas nuclei when brought into close contact.
Diagnostic applications and emerging concepts
All of the techniques yield polarization levels in the range of a few percent to almost 100%, depending on the application. The increasing number of animal applications and clinical studies required adaptations of entire protocols to specific in vivo conditions. The basic configurations of clinical whole body scanners, in particular, and also animal scanners often make hardware adaptations necessary. Further challenges for the translation of initial proof of principle studies mainly include the production of pharmacologically safe hyperpolarized agents and the mastering of in vivo relaxation conditions. Fast encoding of the transiently available magnetization is crucial for making optimum use of the signal enhancement. Based on recent progress, metabolic imaging with tracers such as [1-13C]pyruvate has made it already into clinical applications in oncology.4 Applications based on para-hydrogen are currently exploring various tracers for biomedical applications.5 Hyperpolarized Xe finds an increasing number of applications in lung diseases6 and in basic research to allow for ultra-sensitive imaging of cell surface markers at nanomolar concentrations.7,8Acknowledgements
No acknowledgement found.References
1. Brindle, K. New approaches for imaging tumour responses to treatment. Nat. Rev. Cancer 8, 94–107 (2008).
2. Kurhanewicz, J. et al. Analysis of Cancer Metabolism by Imaging Hyperpolarized Nuclei: Prospects for Translation to Clinical Research. Neoplasia 13, 81–97 (2011).
3. Witte, C. & Schröder, L. NMR of hyperpolarised probes. NMR Biomed. 26, 788–802 (2013).
4. Nelson, S. J. et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 5, 198ra108–198ra108 (2013).
5. Glöggler, S., Colell, J. & Appelt, S. Para-hydrogen perspectives in hyperpolarized NMR. J. Magn. Reson. 235, 130–142 (2013).
6. Walkup, L. L. & Woods, J. C. Translational applications of hyperpolarized 3He and 129Xe. NMR Biomed. 27, 1429–1438 (2014).
7. Rose, H. M. et al. Development of an antibody-based, modular biosensor for 129Xe NMR molecular imaging of cells at nanomolar concentrations. Proc. Natl. Acad. Sci. 111, 11697–11702 (2014).
8. Witte, C. et al. Live-cell MRI with Xenon Hyper-CEST Biosensors Targeted to Metabolically Labeled Cell-Surface Glycans. Angew. Chem. Int. Ed. 54, 2806–2810 (2015).
Figures