Localization (Sequences: semiLASER, PRESS, STEAM, Chemical Shift Displacement)
Ovidiu Cristian Andronesi1
1Radiology, Massachusetts General Hospital, Harvard Medical School, Charlestown, MA, United States
Synopsis
Accurate localization is key for MR spectra quality and metabolites quantification. Metabolites low concentration and multiple frequencies pose more challenges in-vivo MRS than MRI, due to B0 inhomogeneity, insufficient B1, chemical shift displacement, and artifacts from lipids. Volume selection methods based on overlapping slices improves MRS quality by limiting the region of interest to areas where B0 and B1 can be better controlled. Spatial coverage can be improved by more modern approaches where arbitrary volumes can be shaped with parallel transmit, multiple volumes disentangled by parallel imaged, and different contributions to the MRS signal can be modeled in the reconstruction
Purpose
To improve spectral quality for better metabolite quantification. To familiarize the audience with the details of localized MRS acquisition.Methods
Localization methods (PRESS, STEAM, LASER) based on traditional excitation schemes use the overlap of three orthogonal slices to select a volume at the intersection of the orthogonal slices (Figure 1). The slice localization is proportional to the chemical shift of metabolites ($$$\nu$$$) and inverse proportional to the bandwidth (BW) of the RF pulse excitation (the so called chemical shift displacement error, $$$CSDE\sim\nu\diagup BW$$$, Figure 2). Localization methods can be classified based on their excitation bandwidth in non-adiabatic (small BW) and adiabatic (large BW). While the former methods use only amplitude modulation of the RF pulses to achieve slice selection, the later use both amplitude and frequency modulation to effectively increase the RF bandwidth of slice selection. Adiabatic pulses can achieve larger bandwidths when limited by a given maximum amplitude of the RF field (Figure 3). In addition, gradient can also be modulated to achieve even larger bandwidth such as frequency offset independent adiabaticity (FOCI) pulses and gradient offset independent adiabaticity (GOIA) pulses, which in the case of GOIA are able to achieve a BW of 20 kHz with 3.5ms pulse and 0.8 kHz (18 $$$\mu T$$$) maximum amplitude of B1 field by a complex frequency modulation $$$\nu (t) = 1/k\cdot G(t)\int_{0}^{T_{p}}B_1^2(\tau)\diagup G(\tau) d\tau$$$. In addition, adiabatic pulses are effective to compensate for B1 inhomogeneity and can provide more SNR at high magnetic fields.Results
Comparison of localization methods are shown in phantoms and human subjects. The PRESS sequence that uses refocusing pulses of 1.2 kHz has 10%/ppm CSDE at 3T and 23%/ppm CSDE at 7T. A significant reduction of CSDE (0.6%/ppm at 3T, 1.4%/ppm at 7T) and SNR increase can be achieved with LASER using 20 kHz adiabatic pulses (Figure 4). Uniform excitation, sharper transition bands, and no side bands can be demonstrated for adiabatic pulses (Figure 5). This can be performed in human subjects including patients on clinical scanners that are equipped with standard transmit hardware. With more advanced transmit hardware including parallel transmit coils and amplifiers additional degrees of freedom can be exploited in shaping the excitation profile. Compared to the rectangular excitation of PRESS/STEAM/LASER voxels, more conformal excitation can be shaped to an arbitrary anatomical region of interest when parallel transmit is employed.Discussion
MRS acquisition using rectangular volume selection methods is widely used in most clinical and research studies in human subjects. The large availability of sequences, no special hardware requirements and easy of protocol setup make this method a preferred choice for many user groups that do not have technical expertise for more sophisticated approaches. A range of more advance methods are being actively developed to address the limitations of volume selection methods such as reduced spatial coverage and resolution, but currently these methods are not widely available and are still evolving. Hence, understanding and optimizing the mechanisms of volume selection methods is important to achieve the best performance, to eliminate possible sources of artifacts and to obtain adequate spectral quality for reliable metabolite quantification.Acknowledgements
NIH funding K22 CA178269, 1R01CA211080-01. Collaboration with Drs Wolfgang Bogner, Malgorzata Marjanska, Borjan Gagoski, Andre van der Kouwe, and Aaron Hess.References
- Bottomley P.A., Ann NY Sci, 1987, 508:333-348 2.
- Frahm J et al, Magn Reson Med, 1989, 9:79-93 3.
- de la Barre and Garwood, J Magn Reson, 2001, 153: 155-177
- Scheenen TJW et al, Magn Reson Med, 2008, 59:1-6 5.
- Tannus and Garwood, NMR Biomed, 1997, 10:423
- Kinchesh and Ordidge, JMR, 2005, 175:30-43
- Sacolick L et al, Magn Reson Med, 2007, 57:548-553
- Andronesi et al, JMR, 2010, 203:283
- Snyder J et al, Magn Reson Med, 2012, 67:300–309
- Boer V.O. et al, Magn Reson Med 2015, 74:599–606
- Waxmann P et al, NMR Biomed, 2016, DOI: 10.1002/nbm.3558
Figures
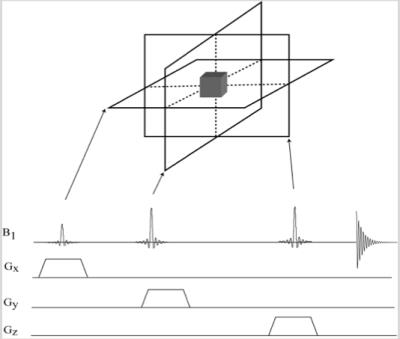
Figure 1. Volume selection with three orthogonal slices. Simple pulse sequence diagram showing RF pulses and slice selective gradients.
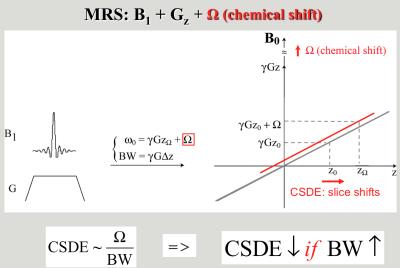
Figure 2. Chemical shift displacement error in slice localization of MRS.
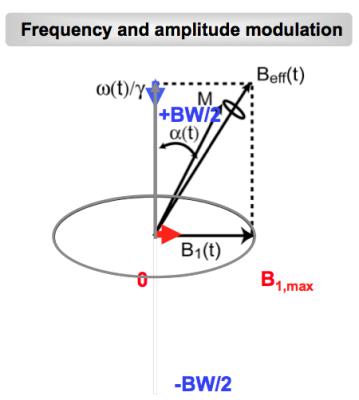
Figure 3. Amplitude and frequency modulation of adiabatic pulses.
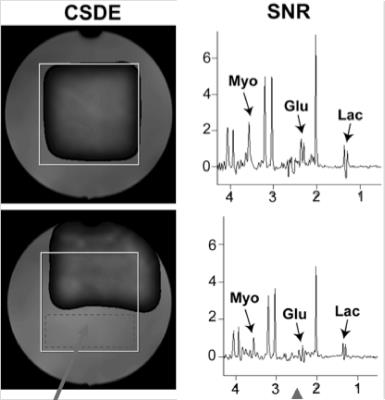
Figure 4. CSDE and SNR comparison with adiabatic (LASER) and non-adiabatic sequences (PRESS).
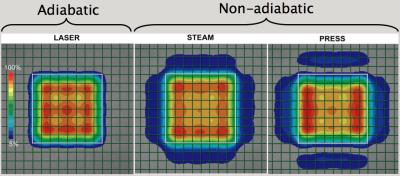
Figure 5. The accuracy of volume selection with adiabatic and non-adiabatic sequences. The selection profile is characterized by the quality of pass-band, transition band, and stop band.