ASL - Data Acquisition
1Radiology, Leiden University Medical Center, Leiden, Netherlands
Synopsis
This talk will outline the basic principles of arterial spin labelling (ASL) data acquisition. The different labeling approaches are compared, the compromise in post labeling delay duration is discussed, why background suppression pulses improve the ASL-signal stability is explained, readout options are described, acquisition parameters are explored and examples of both basic and advanced ASL-techniques are shown.
Introduction
Arterial spin labeling (ASL) measures perfusion using the blood as an endogenous contrast agent by modulating its longitudinal relaxation. Perfusion is defined as the amount of blood delivered to capillary beds in a given tissue per unit time, and cerebral blood flow (CBF) is typically reported as the volume of blood that is delivered to 100 grams of tissue per minute (mL/100 gr/min). Arterial spin labeling was first proposed for perfusion imaging in the early nineties and the technique is still evolving.[1] In a joint effort of the ISMRM Perfusion Study Group and the European ASL in Dementia consortium a consensus paper was written with recommendations for the implementation of ASL for clinical applications.[2]
General ASL concept
In arterial spin labeling
the blood water is magnetically labeled by an RF-pulse or a series of RF-pulses
that inverts or saturates the longitudinal component of the magnetization of
the blood spins. In traditional ASL-methods the labeling occurs in the feeding
arteries at the distal end of the neck region. After a delay in which the
labeled spins are transported further into the vasculature towards the tissue,
an image of the static tissue and the tagged spins that have flown into the
imaging slices is acquired. Subsequently, a control image without labeling is
acquired, which will therefore represent static tissue and the untagged blood.
The subtraction of both image types will remove the static tissue component and
thus only reflects the inflowing tagged spins, which is a measure of the local
tissue perfusion. The quality of a single subtraction image is limited, so
typically 30 averages (or multiple shots for segmented 3D sequences) are
obtained to gain sufficient SNR.
Labeling schemes
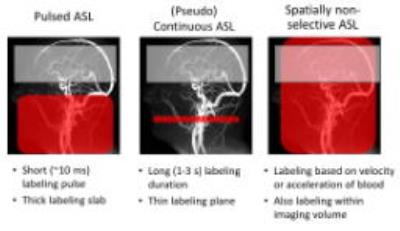
The three main labeling approaches to achieve for perfusion imaging are: A) pulsed ASL (PASL) [3], B) (pseudo)continuous ASL ((p)CASL) [4,5] and C) spatially non-selective ASL (SNS-ASL) [6,7], as schematicaly shown in figure 1.
Pulsed ASL
In PASL, a large volume of blood is inverted with a short inversion pulse of about 10 – 25 ms. There are many different implementations, such as flow alternating inversion recovery (FAIR) [8], Signal Targeting with Alternating Radiofrequency (STAR) [3, 9], Transfer insensitive labeling technique (TILT) [10], and proximal inversion with a control for off resonance effects (PICORE) [11].
The most important pro’s and cons of PASL are:
+ relative
high labeling efficiency
+
low SAR
+
nearly simultaneous inversion of the bolus
- imperfect slice profiles and coil geometry
leading to bolus edges that are not well defined
- unknown temporal width of the bolus of labeled spins
- limited
temporal width and thus relatively low SNR
- tag limited to RF coil length
- sensitive to transit delays
Continuous ASL
In CASL, the magnetization of water molecules in the blood flowing through a thin labeling slice are continuously inverted with a flow-driven adiabatic inversion for 1 – 3 s. In the labeling plane a continuous RF field is applied in the presence of a gradient along the direction of flow. To control for magnetisation transfer effects a double inversion of the spins is performed as the control condition [12].
+ high SNR
+ can use short or separate transmit RF coil
+ well defined bolus
- high SAR, associated with the long pulse
-
difficult to implemented on clinical RF-amplifiers
- low tagging efficiency due to loss of longitudinal magnetization in the
control condition and variability in the blood flow velocity over the heartbeat
- sensitive to transit delays
Pseudo continuous ASL
In pCASL, the long continuous labeling of CASL is replaced by a train of slice-selective, small flip-angle, RF-pulses that gradually invert spins flowing through the labeling plane. The demand on the RF-amplifiers is reduced, therefore enabling easier implementation on clinically used hardware. During the control condition an additional π-phase difference between the odd and even pulses is created in combination with either the same gradient as during the labelling condition (balanced pCASL) or with zero mean gradient (unbalanced pCASL). Both approaches leave effectively the blood magnetization oriented in the direction of the main magnetic field, i.e. no inversion.
+ high SNR
+ can use short or separate transmit RF coil
+ well defined bolus
+ easily implemented on clinical RF-amplifiers
+/- reasonable tagging
efficiency
- high SAR, associated with
the long pulse
- variable labelling efficiency depending on blood flow velocity and
off-resonance effects
- sensitive to transit delays
Spatially non-selective ASL
In spatially non-selective labeling methods the labeling is based on the flow velocity or acceleration of the blood spins. With a velocity/acceleration selective pulse train, based upon motion sensitizing gradients, blood flowing above a chosen velocity/acceleration cut-off is saturated or inverted, regardless of the spatial location. In the control condition, no motion sensitive gradients are used. Since labeling is also effective in the imaging region, the label is created much closer to the tissue compared to traditional ASL methods and does not suffer from long transit delay times, which is especially an advantage in patients with slow or collateral flow.
+ insensitive to transit
delays
- low SNR by labeling using saturation (inversion pulses have recently been
proposed)
- tag limited by RF coil length
Post labeling delay
During the post labeling delay the labeled spins are transported further into the vasculature towards the tissue. The choice for the duration of the PLD is always a compromise: on one hand, the PLD should be long enough for all spins to arrive in the brain tissue, thereby enabling accurate quantification without vascular artifacts caused by label still being present in arteries. On the other hand, the PLD should not be chosen too long, because the labeled spins decay with the longitudinal relaxation time T1 of the compartment in which they reside. If the PLD is too long, all signal will have decayed and no label will be detected [12].
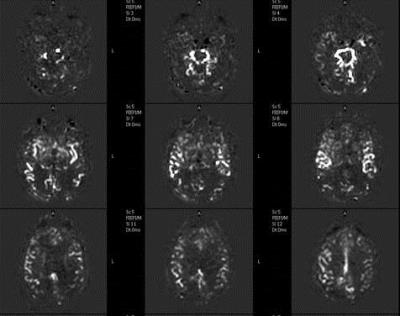
Multi-PLD
Acquiring multiple post labeling delays can be used to estimate the transit times and to obtain a potentially more precise quantification of CBF. In figure 2 an example is shown of ASL maps of a patient with a prolonged arrival times. However, this increases the scan time significantly and also requires more post-processing [13]. Multi-PLD ASL can be acquired by acquiring several single PLD ASL-scans, or by using the Look-Locker approach, which acquires multiple PLD images after a single labeling module [14].
Background suppression
Since the difference in signal intensity between the label and control condition is small, subject movement and physiological pulsations, such as from the respiratory and cardiac cycle, can lead to subtraction errors due to signal fluctuations of the static tissue. Background suppression (BGS) by using spatially non-selective inversion pulses can be employed (often during the PLD) to minimize the signal from static tissue, thereby reducing subtraction errors and significantly improving the ASL-signal stability [15].Readout options
3D readoutCommonly
used 3D segmented methods include 3D multiecho (RARE) stack of spirals
[16,17] and 3D GRASE [18,19]. To reduce too-long
readout times that would lead to blurring, segmentation in the z-direction is
frequently employed in 3D-GRASE readouts.
+ SNR
+ less sensitive to field inhomogeneity than EPI
+ Allows for optimal BGS
- Blurring
in
the z-direction
2D readout
2D single-shot EPI or fast low angle shot (FLASH) readouts are still commonly used ASL read out sequences for the brain [20]. Spin echo sequences provide better image quality than gradient echo sequences, especially lower in the brain, but are also slower. For single-shot 2D readouts an ascending slice order is generally recommended since arrival times are longer in more superior slices and the readout should therefore ‘chase the labeled spins’.
+ widely available on most MR scanners
+ robust against motion
- BGS not optimal in all slices
- longer PLD for later acquired images
- longer scan time than 3D readouts
Advanced ASL-techniques
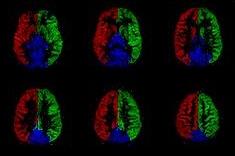
Flow territory mapping
By restricting
the labelling to a single vessel, e.g. by careful positioning of a PASL
labelling slab or by employing a rotating gradient on top of the vessel in
(p)CASL-approaches, one can image the flow territory of that artery [21,22]. An example of regional
perfusion maps is shown in figure 3. Such
information is especially helpful in understanding collateral flow patterns as
well as complicated vascular structures, such as arterio-venous malformations. Another
way to acquire flow territory maps, is by changing the spatial distribution of
the labelling efficiency within the labelling plane and by combining several of
such acquisitions in which the spatial distribution is varied over the
different acquisitions [23]. Clustering algorithms or more advanced post-processing
methods can subsequently be employed to identify several flow territories in
one go.
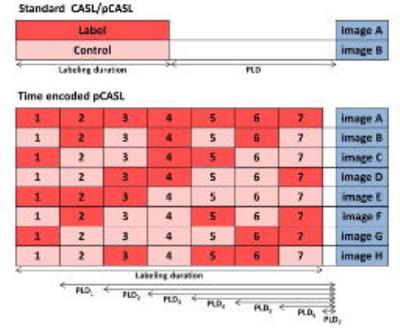
Time-encoded ASL
In traditional ASL methods information about timing is obtained by repeated acquisition with different PLDs and/or labeling durations. In Time encoded pCASL (te-pCASL), also known as Hadamard encoded ASL, signal information at multiple time points is encoded in the labeling by employing a smart labeling scheme. The labeling is spatially selective as in pseudo continuous ASL with a labeling period that ends shortly before imaging, so the waiting time that is usually used for the labeled spins to be transported further into the vasculature is completely filled with labeling. This labeling period is divided into blocks that fluctuate between label and control according to a Hadamard matrix [24]. The number of different images depend on the encoding scheme applied. In the example in figure 4 te-pCASL with a Hadamard-8 labeling scheme is applied, where 7 label and control sub-boli are captured in one image. In post-processing, a concurrent subtraction scheme is applied that renders a perfusion map for each specific block while the signal contribution of all other blocks with different effective PLDs are cancelled out. Importantly, the perfusion image of each block will have the same SNR as a traditional pCASL scans with the same PLD, TR and labeling duration, acquired in the same scan time, i.e. one gets 7 images with the same SNR in the time that one could traditionally only measure one of these images [25].
Acknowledgements
No acknowledgement found.References
1.
Williams, DS, Detre, JA, Leigh, JS,
Koretsky, AP. Magnetic resonance imaging of perfusion using spin
inversion of arterial water. Proc Natl Acad Sci USA. 1992; 89, 212.
2. Alsop, DC., Detre, JA, Golay, X, et al., Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2015;73:102-116
3. Edelman, RR, Siewert, B, Darby, DG, et al., Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192, 513.
4. Kim S-G. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: Application to functional mapping. Magn Reson Med. 1995;34(3):293-301.
5. Dai W, Garcia D, de Bazelaire C, et al. Continuous flow-driven inversion for arterial spin labeling using pulsed radio frequency and gradient fields. Magn Reson Med. 2008;60(6):1488-1497.
6. Wong EC, Cronin M, Wu WC, et al. Velocity-selective arterial spin labeling. Magn Reson Med. 2006;55(6):1334-1341.
7. Schmid S, Ghariq E, Teeuwisse WM, et al. Acceleration selective arterial
spin labeling. Magn Reson Med. 2014;71:191–199
8. Kim S-G. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: Application to functional mapping. Magn Reson Med. 1995;34(3):293-301.
9. Edelman RR, Siewert B, Adamis M, et al. Signal targeting with alternating radiofrequency (STAR) sequences: Application to MR angiography. Magn Reson Med. 1994;31(2):233-238.
10. Golay X, Stuber M, Pruessmann KP, et al. Transfer insensitive labeling technique (TILT): application to multislice functional perfusion imaging. Journal of Magnetic Resonance Imaging. 1999;9(3):454-461.
11. Jahng GH, Zhu XP, Matson GB, et al. Improved perfusion-weighted MRI by a novel double inversion with proximal labeling of both tagged and control acquisitions. Magn Reson Med. 2003;49(2):307-14.
12. Alsop DC, Detre JA. Reduced Transit-Time Sensitivity in Non-invasive Magnetic Resonance Imaging of Human Cerebral Blood Flow. Journal of Cerebral Blood Flow and Metabolism. 1996;16:1236– 1249.
13. Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med. 2012; 67(5):1252–1265
14. Gunther M, Bock M, Schad LR. Arterial Spin Labeling in Combination With a Look- Locker Sampling Strategy: Inflow Turbo-Sampling EPI-FAIR (ITS-FAIR). Magn Reson Med. 2001;46:974–984
15. Garcia DM, Duhamel G, Alsop DC. Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med. 2005;54(2):366-372.
16. Ye FQ, Frank JA, Weinberger DR, McLaughlin AC.Noise reduction in 3D perfusion imaging by attenuating the static signal in arterial spin tagging (ASSIST). Magn Reson Med. 2000;44, 92-100.
17. Vidorreta M, Wang Z, Rodriguez I. Comparison of 2D and 3D single-shot ASL perfusion fMRI sequences. NeuroImage. 2013:66, 662-671.
18. Fernandez-Seara MA, Wang Z, Wang J, et al. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. 2005;54, 1241-1247.
19. Gunther M, Oshio K, Feinberg DA, Single-shot 3D imaging techniques improve arterial spin labeling perfusion measurements. Magn Reson Med. 2005;54, 491-498.
20. Jahng GH, Weiner MW, Schuff N. Improved arterial spin labeling method Applications for measurements of cerebral blood flow in human brain at high magnetic field MRI. Medical Physics. 2007;34(11):4519–4525.
21. Helle M, Norris DG,Rufer S, et al. Superselective Pseudocontinuous Arterial Spin Labeling. Magn Reson Med. 2010;64:777–786
22. Dai W, Robson PM, Shankaranarayanan A, Alsop DC, Modified Pulsed Continuous Arterial Spin Labeling forLabeling of a Single Artery. Magn Reson Med. 2010;64:975–982
23. Hendrikse J, van der Grond J, Lu H, et al. Flow territory mapping of the cerebral arteries with regional perfusion MRI. Stroke. 2004;35(4):882-7.
24. Gunther M. Encoded Continuous Arterial Spin Labeling. Workshop on Cerebral Perfusion and Brain Function: Novel Techniques and Applications; 2007; Salvador da Bahia, Brazil
25. Dai W, Shankaranarayanan A, Alsop DC. Volumetric measurement of perfusion and arterial transit delay using hadamard encoded continuous arterial spin labeling. Magn Reson Med. 2013;69(4):1014-22.
Figures