Introduction to Resting State Functional Connectivity
1Radiology, HMS/MGH/Martinos Center, Charlestown, MA, United States
Synopsis
OBJECTIVES & HIGHLIGHTS
What are the current clinical applications of defining and characterizing resting state networks?
How are static and dynamic resting-state functional connectivity applied clinically?
Does increased acquisition time improve and MRI acceleration techniques dynamic functional connectivity metrics?
Introduction
Resting-state functional magnetic resonance imaging (rs-fcMRI) is a method for determining functional networks in the brain and can be performed without the need of a task. Recent advances of the resting-state fMRI acquisition techniques and analysis suggest it is feasible to evaluate the ictal onset zone in epilepsy, somatomotor system, visual system, language function and perhaps evaluating memory function. Here, we overview the MRI acquisition and applications of resting-state fMRI, with an emphasis of the clinical applications, such as in evaluating patients with epilepsy.MRI Acquisition and Analysis
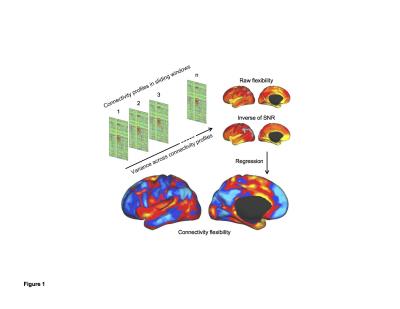
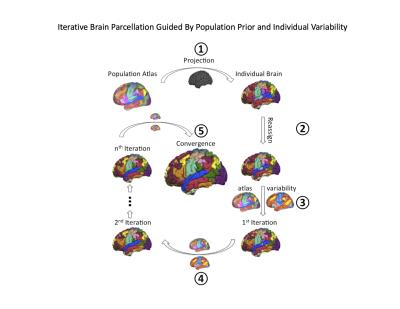
Traditionally, the same fMRI pulse sequences were used as is used by in task fMRI, typically whole head T2* BOLD EPI sequences, with TR = 2-3 seconds. Two major methods exist for analyzing and determining the resting-state networks of rs-fMRI volumes: (1) seed-based approach which uses the temporal correlation between voxels or regions of interest in the brain to determine the networks (2) Data driven methods such as independent component analysis (ICA). Advance analysis includes quantifying the time variation of the time series, such as the flexibility or the temporal variability of the networks (Figure 1). With the development of parallel acquisition, other fast techniques such as simultaneous multislice (sms) or multband (MB) MRI has allowed improved temporal resolution of rsfcMRI. This allows for easier removal of physiologic artifacts such as respiration and cardiac pulsation without the need for extraneous physiologic monitoring (Figure 2). Other approaches including multi echo MRI are also discussed. More recently, automatic and semi-automatic methods have been proposed that identify resting networks with minimal interaction (Figure 3)Applications: The Healthy Brain
The brain spontaneously produces structured activity patterns at rest and sleep, which makes up the human connectome. These spontaneous events cascade through brain systems as oscillations that track anatomy and functional networks. Hence, rs-fcMRI uses these events to unravel the underlying structure of brain systems, both in the healthy and diseased brains. The measurement of spontaneous activity can be used to characterize the organization of the brain or a locus of dysfunction, rapidly, and in individually. From a short fMRI imaging sequence, lasting less than 10 minutes, it is possible to map brain systems in the natural state (or dysfunction), even in an individual.Applications: Clinical Applications
Epilepsy. Here we illustrate clinical applications using pre-surgical planning of epilepsy patients as the primary example. In this application of rs-fcMRI, it is important to both localize and lateralize resting-state networks, as well as define abnormal connectivity. Altered functional connectivity defines abnormal cortex or other brain tissue that can be targeted by neurosurgeons for resection. In epilepsy surgery, hemispheric language dominance, called the language lateralization, has been a major issue especially when a unilateral temporal lobectomy is considered as a surgical management. Intracarotid amobarbital (amytal) procedure (IAP), consisting of administering anesthesia to each hemisphere with amobarbital through a catheter in the internal carotid artery, is the clinical standard technique for determining the language and memory lateralization, however, it is invasive, causing significant risks of stroke and other complications. Non-invasive neuroimaging techniques, including fMRI and MEG, are now frequently used for language lateralization, and successfully reducing the necessity of IAP. These studies activate a subject's neuronal responses generated by performing a language-related tasks such as verb generation or semantic word-processing. The advantage of these neuroimaging techniques is the ability of localization of language function at the lobar or sublobar level in the brain, whereas IAP provides only lateralization information at the hemispheric level, i.e., the left or the right. Classically, Wernicke and Broca areas are considered essential for representing receptive and expressive language function, thus, previous studies investigated the activation in these areas consisting of posterior part of superior/middle temporal gyrus, supramarginal gyrus as well as opercular and triangular parts of inferior frontal gyrus. These anatomical regions are used as the region of interests (ROIs) for determining the lateralization. Laterality index (LI) is calculated as a ratio of the activation amplitude or the volume of the activated cortex in the regions of interest (ROI) between both hemispheres. Various other regions, such as dorsolateral prefrontal cortex and primary motor cortex, also participate in language processing. Presurgical evaluation usually requires mapping the essential language areas, not just participating, therefore employment of optimal ROIs is critical for clinical purposes. Language LI calculation can be done using static rs-fcMRI maps, but memory lateralization may require capturing the temporal variability of the functional connectivity.Acknowledgements
The author would like to acknowledge the contributions of Hesheng Liu, Ph.D., Randy Buckner, Ph.D., and Naoro Tanaka, M.D., Ph.D. for contributions to this presentation.References
1. Wang D, Buckner RL, Fox MD, Holt DJ, Holmes AJ, Stoecklein S, Langs G, Pan R, Qian T, Li K, Baker JT, Stufflebeam SM, Wang K, Wang X, Hong B, Liu H. Parcellating cortical functional networks in individuals. Nat Neurosci. 2015 Dec;18(12):1853-60.
2: Hu ML, Zong XF, Mann JJ, Zheng JJ, Liao YH, Li ZC, He Y, Chen XG, Tang JS. A Review of the Functional and Anatomical Default Mode Network in Schizophrenia. Neurosci Bull. 2017 Feb;33(1):73-84.
3: Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, Van Horn JD. Resting-State Functional Connectivity in Autism Spectrum Disorders: A Review. Front Psychiatry. 2017 Jan 4;7:205.
4: Chiang S, Haneef Z, Stern JM, Engel J. Use of resting-state fMRI in planning epilepsy surgery. Neurol India. 2017;65(Supplement):S25-S33. doi: 10.4103/neuroindia.NI_823_16.
5: Fresco DM, Roy AK, Adelsberg S, Seeley S, García-Lesy E, Liston C, Mennin DS. Distinct Functional Connectivities Predict Clinical Response with Emotion Regulation Therapy. Front Hum Neurosci. 2017 Mar 3;11:86.
6: Cabral J, Kringelbach M, Deco G. Functional Connectivity dynamically evolves on multiple time-scales over a static Structural Connectome: Models and Mechanisms. Neuroimage. 2017 Mar 23.
7: Rosen BR, Huang SY, Stufflebeam SM. Pushing the Limits of Human Neuroimaging. JAMA. 2015 Sep 8;314(10):993-4.
Figures