EPI Acquisition Strategies
1Biomedical Engineering, University of Wisconsin - Madison, 2Medical Physics, University of Wisconsin - Madison, Madison, WI, United States, 3Radiology, University of Wisconsin - Madison, Madison, WI, United States
Synopsis
The presentation provides a summary of echo planar imaging (EPI) acquisition techniques with descriptions of methods used to shorten the acquisition interval to improve imaging performance ( resolution, SNR, distortion, and/or coverage)
Introduction
Introduction: Echoplanar imaging (EPI) is fundamentally and simultaneously old and new in the field of magnetic resonance imaging. Most of the early leaders of MRI were acquiring one Cartesian k-space line per TR (spin warp) at a time while Sir Peter Mansfield was demonstrating how EPI could create ultrafast acquisitions by traversing back and forth with an oscillating readout gradient within a single TR [1]. Initially, there was rampant competition in the field of MRI between these two camps. The invention of RARE spin echo trains, short TR FLASH sequences, and limitations in gradient technology power pushed high resolution anatomical clinical imaging firmly toward spin-warp methods in the late 1980s. But then discoveries in how MR signals could change with variations in perfusion, diffusion, and brain oxygenation levels required the ultrafast and often single shot imaging EPI could provide. These opportunities in the early to mid 1990s served as powerful accelerant for EPI development, with an interesting history provided here [2]. Today spin-warp and EPI acquisition methods complement each other. While spin-warp imaging dominates in high-resolution anatomical imaging, EPI (and its more smoothly varying non-Cartesian relatives like spirals) are the method of choice in functional brain mapping, diffusion-weighted and diffusion tensor imaging, and many applications in real-time MRI.Fundamentals
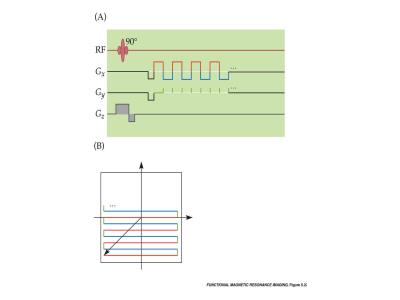
Both the readout and phase-encoding gradients use an initial dephasing pulse to travel to a corner of k-space. After that point, signal acquisition begins as the readout gradient oscillates in a somewhat similar fashion to a RARE sequence. Blips, as shown in Fig. 1A, move the EPI trajectory up one line at time after each readout traversal. Data acquired during the blips is rarely used in the reconstruction, allowing a reconstruction method based on a Cartesian trajectory to be utilized. The blips can be replaced with a small constant Gy gradient, which causes a zig-zag k-space trajectory. As the acquisition points do not naturally fall on a Cartesian grid with this trajectory, a gridding reconstruction or more advance non-Cartesian reconstruction is necessary.
Initially, the rapid switching of the readout gradient in EPI acquisitions was realistically achievable only on relatively unique research scanners with advanced gradient hardware. Today’s gradient technology has progressed to the point that EPI scans are commonplace on most high-field scanners. As peripheral nerve stimulation is always a limitation in human MRI, localized gradient sets still offer the opportunity for higher EPI performance, especially where the geometry of the body supports shorter gradients, like in the head. Phase errors due to system instabilities and chemical shift are somewhat benignly mapped into k-space using spin-warp imaging and so spin-warp imaging is more robust to artifacts than EPI. The back and forth oscillation of the readout gradient in EPI maps phase errors due to system delays and off-resonant signals into more problematic reconstruction issues. Because of this, EPI sequences almost always use lipid suppression from a chemical pre-saturation pulse, spectral spatial pulse, or water-only selective excitation. A thorough description of image reconstruction and related artifact suppression methodologies is provided in the next lecture in this session.
Sequence Variations
Sequence Variations: Gradient-echo EPI (GE-EPI) vs Spin echo EPI (SE-EPI): Just as in spin-warp imaging, EPI acquisitions can be designed as a purely gradient-recalled train of echoes or as a spin-echo sequence. Just as in RARE sequences, the number of echoes in an EPI train is known as the echo train length (ETL) and the spacing between echoes is termed the echo spacing (ESP). In each case, the scheduling of the amplitudes of the Gy gradient dephasing pulse and the individual blip gradients determines when the center of k-space is traversed and thus the effective echo time (TE) of the sequence. In spin-echo EPI, the dephasing pulses are often place prior to the spin-reversal 180 pulse while the oscillating readout gradient is placed entirely after the 180 degree pulse. Signal loss and distortion due to B0 inhomogeneity can be quite problematic in EPI, especially at air-tissue interfaces. . Just as in spin-echo imaging, the signal loss due to B0 magnetic field inhomogeneity is minimized with spin-echo EPI. However, the choice of gradient-recalled over spin-echo EPI is often based upon whether T2* signal decay is desired, as in functional MRI Flyback EPI: Many EPI acquisitions use a flyback readout that essentially throws out every other echo [4]. This scheme lengthens the echo train but removes several of the image reconstruction problems inherent to oscillating delays and oscillating phase errors from off-resonance spins. The discarded echo can be traversed faster as one can use a stronger gradient amplitude than the acquired echoes if the gradient amplitude is available, as it often is. Magnetization Preparation: Magnetization-preparation is desirable to influence image contrast as in many imaging sequences. The use of an inversion recovery pulse (IR-EPI) is often used to add T1 contrast and/or null an unwanted signal species like cerebrospinal fluid. 3D EPI: EPI lends itself to 3D encoding sequences in a fashion similar to spin-warp 3D imaging. The sequencing of additional blips in the slice-encoding dimension needs to be designed into these sequences [5].Shortening the Echo Train Length to Improve Performance
Single-shot vs Mult-shot EPI: As in any gradient-recalled imaging technique acquiring multiple lines of k-space per TR, the signal modulation and signal loss due to T2* ultimately limits SNR, the feasible length of the echo train, and ultimately resolution. One can obtain benefits from a shortened echo train by doing a partial k-space acquisition in the phase-encoding direction. Efforts to shorten the echo train length through faster readout oscillation have the deleterious effect of limiting the period of data acquisition per image, thus lowering SNR. One can also utilize the time during the gradient ramps to shorten the echo spacing. An excellent review of methods for increasing performance is provided here [6] A clever way to effectively limit the echo train length uses in-plane parallel imaging. Instead of having to acquire all phase-encoding lines, parallel imaging allows a reduced number to be acquired. The reconstruction algorithm unwinds the spatial aliasing while the parallel imaging reduction factor reduces the echo train length by an equivalent amount. Like all in-plane parallel imaging methods, a reduction in SNR by the square root of the reduction factor R is suffered. Dividing the EPI sequence into multiple shots with interleaved EPI trajectories effectively shortens the echo train for a given resolution by the number of shots. Usually each echo train in each slot is slightly offset in time from each other to create a smoother mapping of phase errors across k-space. In general, functional imaging methods such as fMRI and diffusion-weighted imaging are quite sensitive to bulk motion between shots, which can be caused by common-place factors such as respiration and cardiac pulsatility. Navigator-based echoes are often acquired in multi-shot EPI acquisitions to provide a of bulk motion for correction in diffusion-weighted EPI sequences [7]. However, fMRI and diffusion studies in the brain continue to be predominantly single-shot sequences. Circular EPI: The echo train length can also be reduced by altering the length of each readout period to sample a circular region rather than a rectangular region [8]. GRASE: The strength of EPI sequence can be combined with the strength of RARE sequences in a method known as GRASE. In GRASE, mini-EPI readout trains are acquired about each RARE spin echo.[9].
Performance Improvement with Simultaneous Multi-slice Imaging
EPI continues to be the workhorse of brain imaging methods centered on the BOLD effect or diffusion-weighting. In general, preparing the spins to be sensitive to BOLD or diffusion and then imaging only a single slice is inefficient. The field of simultaneous multi-slice imaging has created several combination of multi-slice excitation and parallel imaging in the through-plane to accelerate EPI acquisition speed by factors of up to 10. Various methodologies are described in this review article [10].Summary
EPI sequences will continue to be a workhorse in not only clinical brain imaging and neuroscience research, but also in real-time imaging and diffusion imaging throughout the body. Understanding its acquisition fundamentals, described here, and the reconstruction methodologies in the following talk, is very useful to understand the pitfalls, limitations, and exciting applications of EPI.Acknowledgements
No acknowledgement found.References
[1] P. Mansfield, "Multi-planar image formation using NMR spin echoes," J. Phys. C., vol. 10, pp. L55-58, 1977.
[2] M. S. Cohen and F. Schmitt, "Echo planar imaging before and after fMRI: a personal history," Neuroimage, vol. 62, pp. 652-9, Aug 15 2012.
[3] M. A. Bernstein, K. F. King, and Z. J. Zhou, Handbook of MRI Sequences: Elseveir Academic Press, 2004.
[4] J. L. Duerk, O. P. Simonetti, G. C. Hurst, and D. A. Finelli, "Experimental confirmation of phase encoding of instantaneous derivatives of position," Magn Reson Med, vol. 32, pp. 77-87, Jul 1994.
[5] A. W. Song, E. C. Wong, and J. S. Hyde, "Echo-volume imaging," Magn Reson Med, vol. 32, pp. 668-71, Nov 1994.
[6] J. Tsao, "Ultrafast imaging: principles, pitfalls, solutions, and applications," J Magn Reson Imaging, vol. 32, pp. 252-66, Aug 2010.
[7] D. G. Norris, "Implications of bulk motion for diffusion-weighted imaging experiments: effects, mechanisms, and solutions," J Magn Reson Imaging, vol. 13, pp. 486-95, Apr 2001.
[8] A. B. Kerr, J. M. Pauly, B. S. Hu, K. C. Li, C. J. Hardy, C. H. Meyer, et al., "Real-time interactive MRI on a conventional scanner," Magn Reson Med, vol. 38, pp. 355-67, Sep 1997.
[9] K. Oshio and D. A. Feinberg, "GRASE (Gradient- and spin-echo) imaging: a novel fast MRI technique," Magn Reson Med, vol. 20, pp. 344-9, Aug 1991.
[10] D. A. Feinberg and K. Setsompop, "Ultra-fast MRI of the human brain with simultaneous multi-slice imaging," J Magn Reson, vol. 229, pp. 90-100, Apr 2013.