RF
1Institute for Biomedical Engineering, ETH Zurich, Zurich, Switzerland, 2Skope Magnetic Resonance Technologies, Skope Magnetic Resonance Technologies, Zurich, Switzerland
Synopsis
Excitation and detection of NMR on human subjects is intrinsically limited by the interaction of the RF fields and the subject. Therefore most research focuses on the improvements of RF coils. Moving the RF frontend electronics to the coil however has a major impact on the technology and applies to all coils. Furthermore, the lack of measurements during transmission and switching represents the major gap in the detection of the spin state which poses problems for acquisition of short-lived coherences and spin dynamics under RF irradiation. Methods for reduction and omitting this dead time will be discussed.
Introduction
In human MRI the existing technology for RF transmission and reception is mainly limited by subject related factors. On the transmit side, the risk of heating the living tissue limits the applicable power. When receiving, the achievable sensitivity is bound by the thermal noise emitted by the body. Also the spatial degrees of freedom provided by the RF fields inside the body can be accessed and used for enhancing Fourier encoding in reception and transmission by well-known parallel imaging [1-3] and excitation techniques [4, 5]. Therefore, most efforts in research and development of RF equipment target improvements of the units that interface to the subject, the RF coils and arrays. However, alternative approaches for the frontend electronics can significantly facilitate the implementation and alter its application. This is achieved by moving more stages of the RF signal processing towards or even onto the coil. In receive arrays the entire digitization is moved into the bore [6-8] or even directly onto the coil [9]. The obtained data can then be optically or wirelessly transmitted which omits the deployment of RF cabling. This RF cabling and the required protection from common-mode ground currents represent a major design problem, risk, weight and cost factor. This majorly limits the amount of channels that can be built into an array without significantly hampering its usability by its weight and cost. Also the driving electronics of parallel transmission devices have been moved into the bore for improving the stability and coupling situation [10, 11]. However, a major gab in the observation of NMR signals using traditional pulsed systems exists during RF transmission and while switching the T/R state. The temporal separation between transmission and reception and in particular the missing observation during transmission and switching poses no hindrances most of the time. This because spin species that are typically considered under in-vivo conditions must have comparably long transverse relaxation times in order to achieve a usable spatial or spectral resolution on clinical systems. Correspondingly, the time required for spatial or spectral encoding is longer than then required transmission phases and the time required for switching from transmission to reception. From its beginning MRI has been based on pulsed excitation and acquisition schemes. Studying mostly liquid materials with long coherence times, this offers many advantages for sequence design and for the involved hardware compared to continuous wave (CW) NMR schemes. For instance, part of the image or spectral encoding can be deferred to specially designed excitation or refocusing pulses as it is done by slice/volume selective excitations, outer volume or certain fat suppression schemes. Furthermore, individual coherences can be selected etc. For the hardware, the strict separation of transmit and receive intervals significantly reduces the requirement for the dynamic range of the receiver and for the isolation between the paths for transmission and reception signals. SNR optimal acquisition of spin signals during transmission would require the receiver resolving the tiny spin signal down to the level of the thermal noise concurrently with the huge transmission signal leaking into the receiver. Using pulsed schemes the requirement for the receiver is reduced to surviving the transmit pulse and resolving the spin signal only during the read-out. This can be realized by nowadays transmit receive switches (T/R switches) or receive coil detune circuits with low losses and high linearity. Despite all these important advantages, the direct detection of compounds with very short decay times and obviously that of the spin dynamics under RF irradiation is critically hampered by the receiver dead times introduced during transmission and switching. For exploration of short living species the time for detection and spatial encoding is minimal. Consequently, the time in which the NMR cannot be observed becomes relevant [12-14]. These methods rely on exciting and acquiring the NMR signal under a strong gradient for cutting the slewing time, which would prefer an uninterrupted acquisition even during excitation. Also methods which study NMR under [15] or in close conjunction with RF irradiation [16, 17] would greatly benefit from observing nuclear induction during transmission or at least from strictly minimizing the receiver dead time. In this talk, methods and hardware for reducing the blind time during transmission and switching will be discussed along with their implications on the acquisition methods.Gapped acquisitions – Switch swiftly
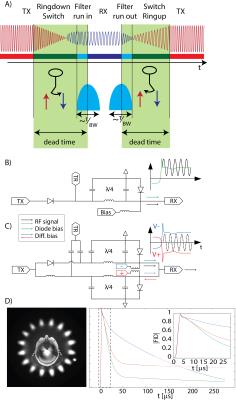
- Rapidly interleaving short transmission pulses with short snippets of acquisitions can be considered as a temporally discretized sampling scheme in the same manner as the pulse waveform and the received signals are typically discretized. The signal is thereby fully captured if the gaps in the acquisition do not violate the applicable sampling criteria. Hence the interleaving frequency rises with the required bandwidth. However, every switching event involves a dead-time for changing the state of the system from transmission to reception and vice-versa. In this switching transient time whether transmission can be performed nor samples can be acquired and the ratio of this dead time on the sequence time rises with the required switching frequency. If the transmission has to be performed in a shorter effective time the power demand scales up. Equally, the SNR efficiency of the acquisition is diminished by the lacking receiver time. Hence a reduction in the switching dead time is key for pushing the boundaries of these methods. The most prominent example here are certainly stochastic resonance approaches [16-18] and SWIFT [19] that are typically performed under strong gradients to save the time for gradient switching preventing the decay of the spin signal. Here the gaps in the acquisition and in the transmission must be short enough such that the sampling introduced by the gaps fulfills the Nyquist criterion. For acquisitions with strong read gradients - as required for resolving short leaving coherences and concomitantly high bandwidths - these gaps have to be shortened. Similarly PETRA sequences [14] have to resign to a time consuming single point encoding within the k-space covered during the excitation pulse and the switching dead-time. Using ZTE [13] reconstruction this sequence overhead can be omitted, however the gap length becomes very critical since the conditioning scales with the k-space gap size and only a few Nyquist distances can be tolerated at all. Concluding the above, the only parameter that does not represent a trade-off in gapped acquisition is reducing the dead time for switching between the two states. This represents a challenge on various points of the RF chains and consequently the available dead-times on human MRI system reside between 10-100 µs and about 4 µs on cutting-edge solid state NMR high resolution systems. In particular the achievable transient times on human system is too long for efficient application of most of the methods mentioned above. First, employed coils are tuned to a narrowband resonance which first has to ring down. There are however methods to reduce this ring down by hardware measures [20] or dedicated RF pulses [21]. Furthermore, the employed switches have to change and settle their state very quickly. This is a particular problem due to the involved very high power levels in the transmit phase. In order to achieve the required power handling, low signal losses and linearity, comparably slowly switching PIN diodes have to be employed. In order to speed-up their switching transients higher control voltages and currents have to be applied which in turn creates large spikes on the RF lines. These spikes saturate the receiver which requires then again time to recover to a stable operation. This problem has recently been resolved by a new type of RF switch topology inherently suppressing the switching spikes [22] enabling switching below 1 µs. Finally, the filters involved in the receive chain have to settle. Fast settling however competes directly with a high frequency selectivity as required by heterodyne receivers. Therefore broadband receivers can offer much shorter transient times compared to narrow-band systems by moving part of the filtering to the final reconstruction [23] enabling acquisition of the first samples 1 µs after the excitation pulse. In conclusion, switching from transmission to reception can nowadays be performed within a microsecond which is typically below the output sampling rate of the acquisition under the strongest regularly available gradients. Hence, the limiting factor for resolution of very shortly relaxing compounds becomes the available gradient strength and not the switching rate, at least for methods such as ZTE [13] and PETRA [14].
Continuous wave NMR
First NMR spectroscopic experiments were carried out with continuous wave (CW) operation which means that the sample was constantly irradiated with RF power at a fixed frequency and the received signal was recorded at the same time. For this, a very high isolation has to be established between the transmitter and the receiver. Furthermore, the remnant coupling has to be stable, otherwise the leakage background signal is modulated and spread over the spectrum. This was achieved by using isolation bridges and sweeping the B0 field instead of the RF frequency. But rarely live samples have been studied which can alter the coupling by their breathing or other motion. Only recently, implementations of such CW NMR based imaging have been shown on human MRI systems [24]. Transmit and receive paths were decoupled using a highly isolating hybrid and the remnant leakage was finally corrected out of the received data [25]. However, only minute power could be applied in this scheme. More recently transmit and receive coils have been isolated using parallel transmit systems [26]. The idea is to drive several transmit channels such that the coupling into the receive channel is cancelled out. However, in these approaches it has to be taken into account that also the transmit channels have a final dynamic range and accuracy. Noise and spurs introduced on the different channels can couple to the receiver and diminish the quality of the acquired signal.Slow modulation
An elegant way to suppress stationary leakages out of a CW NMR setup is to modulate the NMR by a temporally varying main field [27]. By this, the frequency of the adsorption of the nuclear resonance shifts in a pre-determined fashion. This can then be detected as a slight change in the electrical parameters of the transmit coil by use of lock-in amplifiers. The main drawback of these approaches is that they are bound to the NMR steady state condition, which means that the spins must fully relax under the RF irradiation. Therefore, the sweeping frequency should be smaller than the NMR spectral width. This intrinsically limits the sweeping speed and hence results in comparably long acquisitions.Multi-photon, Sideband Modulation
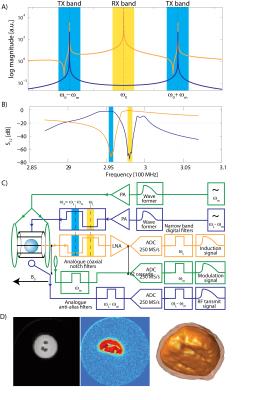
In contrast to slow modulation fast modulation i.e. modulation of the NMR at frequencies substantially higher than the width of the encountered spectra can be employed. Here, the sample is irradiated with RF power with at least two frequencies that add up to the NMR frequency [28]. This forms a higher order resonance condition which finally excites the spin system. The process employs the non-linearity of the spin system in order to mix the incident frequencies to the NMR frequency. The spin system then acts similarly as an RF frequency mixer or non-linear optical media. Since the RF frequencies merge to a third, the process can be seen as two photons exciting the nuclear Zeeman transition together. Hence this technique is frequently called multi-photon excitation which emphasizes close analogies to multi-photon techniques routinely applied in optics. However, in the classic picture it can be seen as one of the applied RF frequencies modulates the NMR out of the band of the Larmor frequency, and the second frequency hits this sideband (see Fig. 2). Therefore this technique used to be called sideband NMR or sideband excitation and was frequently applied for the construction of frequency locks for NMR spectrometers [29] and is still used to obtain correlation spectra from electron paramagnetic resonances (EPR). Recently also multi-photon NMR imaging acquiring during transmission was demonstrated [30, 31]. The main difference to the methods described above is that the RF signals for transmission and reception are in two separated frequency bands. Therefore, the two signal chains can be isolated from each other using frequency selective, high power capable analogue and highly selective digital filters. This provides a very stable and high isolation and direct signal leakage can be almost entirely suppressed. However, non-linear behavior of the setup can introduce a modulated signal and is required to be kept as low as possible. The main drawback of these techniques is that their power efficiency is intrinsically low. The penalty scales with the modulation frequency. Therefore the modulation frequency has to be kept as low as possible. This in turn requires filters with very steep frequency transition bands and consequently high Q components. For filtering high power signals coaxial cavity or helical resonator notch filters, as frequently used in RF repeater systems, proved to be very efficient. After first filtering the lower power levels could well be handled by surface acoustic wave filters or ceramic resonator topologies which however imply a higher design and implementation effort. The main advantage of the multi-photon or sideband approaches is that a very high and stable isolation can be achieved allowing comparably high transmit powers to be applied resulting in fast in-band nutation [32]. However, even with very steep filters and modulation frequencies as low as 2.25 MHz the power penalty compared to an in-band excitation is very high.Longitudinal detection
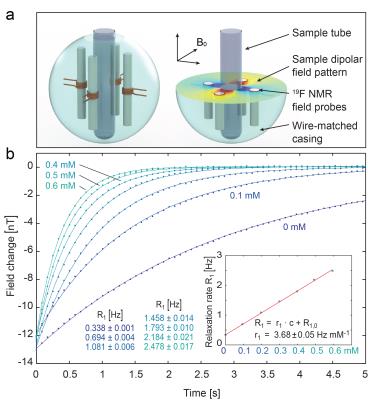
Most compounds with very short transverse relaxation times exhibit comparably long time scales for longitudinal relaxations. Hence a detection of the magnetic field longitudinally induced by the spins’ dipole moment would be a very elegant way to circumvent the necessity to detect the transverse magnetization at all. However, the field modulation induced by the spins are very tiny and compete in longitudinal direction with the fields induced by the bulk susceptibility of the material introducing a few substantial experimental problems. However, the induced field of several nanotesla can be detected at high field using NMR magnetometers [33] (see Fig. 3) or in very close vicinity to the sample using magnetic force detection [34]Acknowledgements
No acknowledgement found.References
1. M.A. Griswold, P.M. Jakob, R.M. Heidemann, M. Nittka, V. Jellus, J.M. Wang, B. Kiefer, and A. Haase, Generalized Autocalibrating Partially Parallel Acquisitions (GRAPPA). Magn.Reson.Med., 2002. 47(6): p. 1202-1210 DOI: 10.1002/mrm.10171.
2. K.P. Pruessmann, M. Weiger, M.B. Scheidegger, and P. Boesiger, SENSE: sensitivity encoding for fast MRI. Magn Reson Med, 1999. 42: p. 952-962.
3. D.K. Sodickson and W.J. Manning, Simultaneous acquisition of spatial harmonics (SMASH): Fast imaging with radiofrequency coil arrays. Magn.Reson.Med., 1997. 38(4): p. 591-603.
4. U. Katscher, P. Boernert, C. Leussler, and J.S. van den Brink, Transmit SENSE. Magn Reson Med, 2003. 49(1): p. 144-150.
5. Y. Zhu, Parallel excitation with an array of transmit coils. Magn Reson Med, 2004. 51(4): p. 775-784.
6. C. Possanzini, P.R. Harvey, k. Ham, R. Hoogeveen, and M. Stoesz, dStream architecture: the digital revolution in MRI, P.M. Systems, Editor 2011.
7. J. Reber, J. Marjanovic, D.O. Brunner, A. Port, and K.P. Pruessmann. Scalable, In-Bore Array Receiver Platform for MRI. in Proc Intl Soc Magn Reson Med. 2016. Singapore. p. 2170.
8. W. Tang, W. Wang, W. Liu, Y. Ma, X. Tang, L. Xiao, and J.-H. Gao, A home-built digital optical MRI console using high-speed serial links. Magn Reson Med, 2015. 74(2): p. 578-588 DOI: 10.1002/mrm.25403.
9. B. Sporrer, L. Wu, L. Bettini, C. Vogt, J. Reber, J. Marjanovic, T. Burger, D.O. Brunner, K.P. Pruessmann, G. Tröster, and Q. Huang, A Sub-1dB NF Dual-Channel On-Coil CMOS Receiver for Magnetic Resonance Imaging, in IEEE ISCC2017: San Francisco, CA, USA. p. 27.4s.
10. L. DelaBarre, D.P. Myer, and J.T. Vaughan. Muti-Channel, In-bore Power Amplifiers for Multi-channel Coil at 7T. in Proc Intl Soc Magn Reson Med. 2013. Salt Lake City, USA. p. 0726.
11. N. Gudino, Q. Duan, J.A. de Zwart, J. Murphy-Boesch, S.J. Dodd, H. Merkle, P. van Gelderen, and J.H. Duyn, Optically controlled switch-mode current-source amplifiers for on-coil implementation in high-field parallel transmission. Magn Reson Med, 2015: p. n/a-n/a DOI: 10.1002/mrm.25857.
12. C. Bergin, J. Pauly, and A. Macovski, Lung Parenchyma: Projection Reconstruction MR Imaging. Radiology, 1991. 179(3): p. 771-781.
13. M. Weiger, D.O. Brunner, M. Tabbert, M. Pavan, T. Schmid, and K.P. Pruessmann, Exploring the bandwidth limits of ZTE imaging: Spatial response, out-of-band signals, and noise propagation. Magn Reson Med, 2014. 74(5): p. 1236-1247 DOI: http://dx.doi.org/10.1002/mrm.25509.
14. D.M. Grodzki, P.M. Jakob, and B. Heismann, Ultrashort echo time imaging using pointwise encoding time reduction with radial acquisition (PETRA). Magn Reson Med, 2012. 67(2): p. 510-518 DOI: 10.1002/mrm.23017.
15. A. Borthakur, E. Mellon, S. Niyogi, W. Witschey, J.B. Kneeland, and R. Reddy, Sodium and T(1ρ) MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed, 2006. 19(7): p. 781-821 DOI: 10.1002/nbm.1102.
16. R.R. Ernst, Magnetic resonance with stochastic excitation. Journal of Magnetic Resonance (1969), 1970. 3(1): p. 10-27 DOI: http://dx.doi.org/10.1016/0022-2364(70)90004-1.
17. D. Ma, V. Gulani, N. Seiberlich, K. Liu, J.L. Sunshine, J.L. Duerk, and M.A. Griswold, Magnetic resonance fingerprinting. Nature, 2013. 495(7440): p. 187-192 DOI: http://www.nature.com/nature/journal/v495/n7440/abs/nature11971.html#supplementary-information.
18. B. Blümich, White noise nonlinear system analysis in nuclear magnetic resonance spectroscopy. Progress in Nuclear Magnetic Resonance Spectroscopy, 1987. 19(4): p. 331-417 DOI: http://dx.doi.org/10.1016/0079-6565(87)80011-0.
19. D. Idiyatullin, C. Corum, J.-Y. Park, and M. Garwood, Fast and quiet MRI using a swept radiofrequency. Magn Reson, 2006. 181(2): p. 342-349 DOI: http://dx.doi.org/10.1016/j.jmr.2006.05.014.
20. A.S. Peshkovsky, J. Forguez, L. Cerioni, and D.J. Pusiol, RF probe recovery time reduction with a novel active ringing suppression circuit. Journal of magnetic resonance (San Diego, Calif. : 1997), 2005. 177(1): p. 67-73 DOI: 10.1016/j.jmr.2005.07.004.
21. D.I. Hoult, Fast recovery, high sensitivity NMR probe and preamplifier for low frequencies. Rev. Sci. Instrum., 1979. 50(2): p. 193 DOI: 10.1063/1.1135786.
22. D.O. Brunner, L. Furrer, M. Weiger, W. Baumberger, T. Schmid, J. Reber, B.E. Dietrich, B.J. Wilm, R. Froidevaux, and K.P. Pruessmann, Symmetrically biased T/R switches for NMR and MRI with microsecond dead time. Magn Reson, 2016. 263: p. 147-155 DOI: http://dx.doi.org/10.1016/j.jmr.2015.12.016.
23. M.A. Bernstein, X.J. Zhou, J.A. Polzin, K.F. King, A. Ganin, N.J. Pelc, and G.H. Glover, Concomitant gradient terms in phase contrast MR: Analysis and correction. Magn Reson Med, 1998. 39(2): p. 300-308 DOI: 10.1002/mrm.1910390218.
24. D. Idiyatullin, S. Suddarth, C.A. Corum, G. Adriany, and M. Garwood, Continuous SWIFT. Magn Reson, 2012. 220: p. 26-31 DOI: http://dx.doi.org/10.1016/j.jmr.2012.04.016.
25. S.-M. Sohn, J.T. Vaughan, R.L. Lagore, M. Garwood, and D. Idiyatullin, In vivo MR imaging with simultaneous RF transmission and reception. Magn Reson Med, 2016. 76(6): p. 1932-1938 DOI: 10.1002/mrm.26464.
26. A.C. Özen, M. Bock, and E. Atalar, Active decoupling of RF coils using a transmit array system. Magn Reson Mater Phy, 2015. 28: p. 565–576 DOI: 10.1007/s10334-015-0497-0.
27. D.J. Lurie, S.J. McCallum, J.M.S. Hutchison, and m. Aleccit, Continuous-wave NMR imaging of solids. Magn Reson Mater Phy, 1996. 4(1): p. 77-81 DOI: 10.1007/BF01759783.
28. E.M. Krauss and S. Vega, Four-field excitation of multiphoton NMR resonance in spin I=1/2. Phys Rev A, 1986. 34(1): p. 333-359. 29. F.W. van Deursen and A. P, An automatic lock holder for the Varian HA-100 NMR spectrometer. J Phys E, 1972. 5: p. 568-570.
30. D.O. Brunner, B.E. Dietrich, M. Pavan, and K.P. Prüssmann. MRI with Sideband Excitation - Application to Continuous SWIFT. in Proc Intl Soc Magn Reson Med. 2012. Melbourne, Australia. p. 150.
31. D.O. Brunner, B.E. Dietrich, M. Pavan, and K.P. Prüssmann. Fast Reconstruction for RF Monitored Sweep Imaging with Sideband Excitation. in Proc Intl Soc Magn Reson Med. 2013. Salt Lake City, UT, USA. p. 562.
32. C.A. Michal, S.P. Hastings, and L.H. Lee, Two-photon Lee-Goldburg nuclear magnetic resonance: Simultaneous homonuclear decoupling and signal acquisition. The Journal of Chemical Physics, 2008. 128(5): p. 052301 DOI: 10.1063/1.2825593.
33. S. Gross, C. Barmet, B.E. Dietrich, D.O. Brunner, T. Schmid, and K.P. Pruessmann, Dynamic nuclear magnetic resonance field sensing with part-per-trillion resolution. Nature Communications, 2016. 7: p. 13702 DOI: 10.1038/ncomms13702.
34. L.A. Madsen, G.M. Leskowitz, and D.P. Weitekamp, Observation of force-detected nuclear magnetic resonance in a homogeneous field. Proceedings of the National Academy of Sciences of the United States of America, 2004. 101(35): p. 12804-12808 DOI: 10.1073/pnas.0405232101. o
Figures