MR Fingerprinting
1Case Western Reserve University
Synopsis
Magnetic Resonance Fingerprinting is an emerging technique for the simultaneous collection of T1 and T2 maps. Cardiac MRF is also possible, and can be used to make multiparametric maps of one or more 2D slices of the heart in 5 to 10 heartbeats.
Magnetic Resonance Fingerprinting (MRF) has recently emerged as a technique that can simultaneously map T1, T2, and proton density values in a single short (<10s) scan [1]. In MRF, the pulse sequence parameters (typically the flip angles and TRs) are varied during the scan, which prevents the magnetization from reaching a steady state. Tissues with different underlying T1 and T2 values will exhibit signal evolutions that are different from one another. These signal evolutions are complex and difficult to fit to a simple signal model, such as an exponential recovery or decay, as would be done in conventional parameter mapping. Instead, the experimental signal evolution in each pixel is matched to a dictionary of possible signal evolutions calculated using the Bloch equations, knowledge of the pulse sequence, and an input range of T1 and T2 values. While it would be possible to perform tissue property mapping in this way using fully-sampled data, the acquisition time would be long (approximately several minutes for a 2D scan). In MRF, a highly undersampled spiral trajectory is employed to dramatically reduce the scan time. As long as the aliasing artifacts produced by the undersampling are incoherent, pattern matching can still be used to extract the underlying T1 and T2 values despite the additional interference.
MRF has been used to generate T1 and T2 maps in the brain [1,2], prostate [3], and body [4] in both 2D and 3D. While first used to map relaxation values, MRF can be employed to map any tissue property to which a sequence is sensitive, diffusion [5], perfusion [6,7], partial volume effects [8], and microvascular parameters such as blood oxygenation [9] . However, MRF relies on the idea that the underlying tissue properties do not change during the acquisition, and thus the tissues cannot be in continual motion during the scan. While the reconstruction is less sensitive to motion that occurs in one concentrated part of the acquisition, it is challenging to take into account the affects of through-plane motion or motion that happens throughout the scan.
These limitations have required specialized developments for
cardiac MRF (cMRF) [10], which will be discussed in this presentation. Firstly, due to the field
inhomogeneities, a FISP-based [11] sequence is employed instead of the originally
suggested bSSFP sequence. This
leads to a reduced signal level but also relative insensitivity to
off-resonance effects. Secondly,
both cardiac and respiratory motion present challenges to cMRF due to the
through-plane and continuous motion.
In this implementation of cMRF, magnetization is excited and data are
collected during a breathhold and only in diastole. In order to determine the signal time courses for pattern
matching, the subject’s heart rate, and thus the exact timing of the scan, is
used as an input into the Bloch simulation to determine a subject-specific dictionary. This solution leads to another set of
difficulties, namely the “start-and-stop” nature of the scan, which results in T1
recovery during the non-imaging periods.
In order to maintain differences in signal evolutions for tissues with
different T1 and T2 values, both inversion pulses and variable
T2-preparation modules are used during the scan. Because the scan time is limited to the
length of a breathhold, the TR is held constant at a minimal value such that
the number of images collected can be maximized. Finally, the flip angle is varied but kept low to minimize
effects from imperfect slice profiles and B1+
inhomogeneities, which could lead to errors in the T1 and T2
maps. The resulting cMRF sequence
is a 10- to 15-heartbeat scan performed in diastole where the following
5-heartbeat pattern is repeated (either two or three times): Inversion, no prep, T2 prep
(30ms), T2 prep (50ms), T2 prep (80ms). The spatial resolution is typically set
to 1.6x1.6x8 mm3, with a matrix size of 192x192 and a scan window of
250ms. The cMRF sequence has been
tested in phantom and normal volunteer studies, and the results for both T1
and T2 agree with literature values and maps made using standard
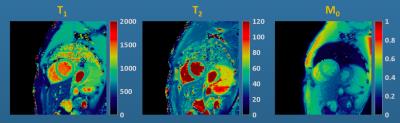
cardiac relaxometry sequences (MOLLI and bSSFP T2) at 1.5 and 3T [10]. An example set of maps from a normal volunteer is shown in Figure 1.
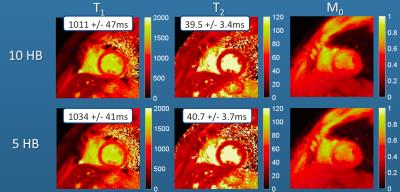
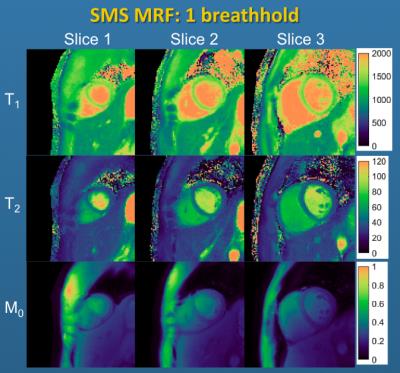
Several additional modifications to the cMRF technique have recently been proposed. The first is the use of a compressed sensing like reconstruction, known as Sparse MRF [12], instead of the basic inner product matching for the generation of parameter maps. This iterative reconstruction can reduce the number of images required for a given precision in the cMRF measurements; concretely, it can be used to reduce the number of heartbeats needed from 10 to 5, thereby reducing the total breathhold length (see Figure 2). Sparse MRF has also enabled the collection of time courses from several cardiac slices simultaneously in combination with SMS imaging. SMS cMRF has been used to collect T1 and T2 maps from 3-6 separate slices in 10 heartbeats [13], as shown in Figure 3.
In conclusion, MRF is an emerging technique for the simultaneous collecting of T1 and T2 maps. Cardiac MRF is also possible, and can be used to make multiparametric maps of one or more 2D slices in 5 to 10 heartbeats. Because the signal time courses are matched to relaxation parameters using direct Bloch equation simulations, changing heart rates and arrhythmias can be incorporated in the MRF dictionary, which may lead to fewer errors in the parameter maps than standard imaging sequences.
Acknowledgements
This work has been supported by Siemens, CAREER NSF/CBET 1553441, NIH/NHLBI R01HL094557, and NIH/NIDDK R01DK098503.References
[1] Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature. 2013 Mar 14;495(7440):187-92. [2] Badve C, Yu A, Dastmalchian S, Rogers M, Ma D, Jiang Y, Margevicius S, Pahwa S, Lu Z, Schluchter M, Sunshine J, Griswold M, Sloan A, Gulani V. MR Fingerprinting of Adult Brain Tumors: Initial Experience. AJNR Am J Neuroradiol. 2016 Dec 29. doi: 10.3174/ajnr.A5035. [3] Yu AC, Badve C, Ponsky LE, Pahwa S, Dastmalchian S, Rogers M, Jiang Y, Margevicius S, Schluchter M, Tabayoyong W, Abouassaly R, McGivney D, Griswold MA, Gulani V. Development of a Combined MR Fingerprinting and Diffusion Examination for Prostate Cancer. Radiology. 2017 Feb 10:161599. doi: 10.1148/radiol.2017161599. [4] Chen Y, Jiang Y, Pahwa S, Ma D, Lu L, Twieg MD, Wright KL, Seiberlich N, Griswold MA, Gulani V. MR Fingerprinting for Rapid Quantitative Abdominal Imaging Radiology. 2016 Apr;279(1):278-86. [5] Jiang Y, Wright KL, Seiberlich N, Gulani V, Griswold MA. Simultaneous T1, T2, diffusion and proton density quantification with MR fingerprinting. In: In proceedings of the 22nd annual meeting of ISMRM meeting & exhibition in Milan, Italy. 2014. p. 28. [6] Su P, Mao D, Liu P, Li Y, Pinho MC, Welch BG, Lu H. Multiparametric estimation of brain hemodynamics with MR fingerprinting ASL. Magn Reson Med. 2016 Dec 26. doi: 10.1002/mrm.26587. [7] Wright KL, Ma D, Jiang Y, Gulani V, Griswold MA, Luis H-G. Theoretical framework for MR fingerprinting with ASL: simultaneous quantification of CBF, transit time, and T1. In: In proceedings of the 22nd annual meeting of ISMRM meeting & exhibition in Milan, Italy. 2014. p. 417. [8] Deshmane AV, Ma D, Jiang Y, et al. Validation of Tissue Characterization in Mixed Voxels Using MR Fingerprinting. In: In proceedings of the 22nd annual meeting of ISMRM meeting & exhibition in Milan, Italy. 2014. p. 0094. [9] Christen T, Pannetier NA, Ni WW, Qiu D, Moseley ME, Schuff N, Zaharchuk G. MR vascular fingerprinting: A new approach to compute cerebral blood volume, mean vessel radius, and oxygenation maps in the human brain. Neuroimage. 2014 Apr 1;89:262-70. [10] Hamilton J, Jiang Y, Chen Y, Ma D, Lo W-C, Griswold M, Seiberlich N. MR Fingerprinting for Quantification of Myocardial T1, T2, and Proton Spin Density Magn Reson Med. 2016 Apr 1. doi: 10.1002/mrm.26216. [11] Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015 Dec;74(6):1621-31. [12] Hamilton JI, Jiang Y, Ma D, Chen Y, Pawha S, Lo W-C, Batesole J, Griswold MA, Seiberlich N. Low Rank Compressed Sensing Reconstruction for More Precise Cardiac MRF Measurements. In: Proceedings of the 25th Annual Meeting of ISMRM, Honolulu, HI, USA. ; 2017. p. 554 [13] Hamilton JI, Jiang Y, Ma D, Lo W-C, Griswold MA, Seiberlich N. Cardiac MR Fingerprinting with Simultaneous Multi-Slice Imaging for T1 and T2 Quantification. In: 20th Annual Society for Cardiovascular Magnetic Resonance Scientific Sessions, Washington, D.C. ; 2017.Figures