Fat Water Separation: Applications in MSK imaging
1Karolinska Institutet
Synopsis
Dixon’s fat/water separation method offers fat suppression based on chemical shift, insensitive to main field inhomogeneity, which is desirable in musculoskeletal MRI when imaging off-center, at anatomical sites with strong patient induced field inhomogeneity, or near metallic implants. A single acquisition additionally yields non fat suppressed and fat only-images, providing additional information that can potentially replace conventional images, in particular imaging both with and without spectral fat saturation. The method is compatible with both spin echo and gradient echo sequences with various contrast weighting. The prolonged acquisition time comes with increased SNR, providing capacity for compensation using other acceleration techniques.
Background
In the musculoskeletal system, the most abundant types of molecules containing hydrogen are water, fat (triglyceride), phospholipids, and proteins such as collagen. Neither the phospholipids found in membrane bi-layer structures, nor the low-mobility proteins are directly visible by standard MR sequences, leaving only water and fat as contributors to the signal in proton MRI1.
In many cases, the water signal carries better information about pathological processes, while the fat signal is useful for anatomical reference. In some situations however, the fat signal in itself can be indicative of pathology. As examples, decreased fat signal may reveal abnormalities in the bone marrow, and increased fat signal can indicate fatty infiltration in skeletal muscle. Also, the fat content can be of interest to characterize lesions containing fat2.
Compared to the water signal, the appearance of fat differs in several aspects for two reasons: First, the size of the fat molecules will increase the number of interactions near the Larmor frequency, effectively shortening the T1 relaxation of fat rich tissue. This makes fat appear bright on T1-weighted images3. Second, there is a small difference in resonance frequency between water and fat protons, about 3.4 ppm of the Larmor frequency. This so-called chemical shift arises since fat hydrogen are mostly bound to Carbon, which is less electronegative than Oxygen. This will give fat hydrogen nuclei more electron shielding, resulting in a slightly slower precession frequency. During signal readout, the chemical shift will resemble the shift induced by the gradient, causing the fat signal to appear further down the read gradient than the water signal. This spatial mismapping is known as chemical shift displacement artifact1. At certain echo times in gradient echo sequences, the relative dephasing between water and fat will result in a signal cancellation. This will appear as dark interfaces between water rich and fat rich tissue, known as Signal cancellation artifact or India ink artifact4. Compared to most other tissues in the musculoskeletal system, fatty tissue also has relatively long T2 relaxation time, giving it a bright appearance on T2-weighted and intermediate-weighted (PD-weighted) images.
Since the fat signal is bright on most images and associated with artifacts, while information about pathology is typically found in the water signal, it is often desirable to suppress the fat3. In musculoskeletal MRI, fat suppression is often used with PD-weighted images, T2-weighted images, and contrast enhanced T1-weighed images.
Among the many available methods for fat suppression, fat/water separation – also known as the Dixon method5 – is of special interest due to its many advantages, including the ability to correct for B0 field inhomogeneity. This method separates the signal components into a water-only and a fat-only image. The water-only image works exactly like a fat suppressed one, while the fat-only image provides additional information about the location of fat.
Methods
The most common MRI fat suppression methods are either based on the short T1 of fatty tissue, or on the chemical shift of protons in fat molecules.
Short Time Inversion Recovery
Inversion recovery sequences have an inversion pulse before the excitation pulse, flipping the tissue magnetization from its equilibrium state on the positive longitudinal axis to the negative longitudinal axis. Due to T1 relaxation, the magnetization will then gradually recover to equilibrium, passing through the origin corresponding to zero magnetization. If the excitation pulse is played exactly when a tissue’s magnetization crosses zero, that tissue will have zero signal in the image. Since fatty tissue has fast T1 recovery, nulling the signal from fatty tissue requires a short time between inversion and excitation. It is hence called Short Time Inversion Recovery (STIR)6. STIR offers homogeneous fat suppression insensitive to B0 field inhomogeneity. STIR images have a special kind of “inverted” T1 contrast, and may additionally be T2-weighted if the echo time is long. This special contrast makes STIR very sensitive to edema. STIR should not be used after contrast agent has been administered, since all tissue with T1 similar to fatty tissue will be suppressed, including contrast enhanced tissue2.
Spectral fat saturation
The idea of spectral fat saturation (“fatsat”) is to add a prepulse that saturates the fat signal before the actual sequence kernel. This prepulse is applied repeatedly during the acquisition. The saturation pulse is a chemical shift selective pulse (CHESS)7, meaning it must be narrowband (long duration) and played with no gradient on (non slice selective). After the CHESS pulse selectively has tipped the fat magnetization into the transverse plane, a spoiler gradient destroys the signal. Spectral fat saturation is easy to combine with most pulse sequences, does not affect the weighting, and can be combined with contrast enhancement. The main drawback is its sensitivity to B0 field inhomogeneity, which can lead to incomplete fat saturation (if the inhomogeneity shift is downfield), or in the worst case unintentional suppression of the water signal (if the inhomogeneity shift is upfield). Even with a perfect main magnet, B0 inhomogeneity is unavoidable since it will also be induced by susceptibility differences between the patient’s tissues, and even more by susceptibility differences between the patient and the surrounding air.
There also exist hybrid techniques that combine the principles of Spectral fat saturation and STIR, such as Selective Partial Inversion Recovery (SPIR)8 and Spectral Inversion At Lipids (SPECIAL)9.
Water excitation by spectral-spatial pulses
These techniques combine spectrally selective excitation of water with slice selection10. This can be achieved by applying several slice selective pulses with an interpulse delay to allow 180° dephasing of the water and fat magnetization (e.g. 1.1 ms at 3T), the simplest example being two 45° pulses. The first pulse flips both water and fat by 45° towards the transverse plane. During the delay, the fat magnetization rotates 180° about the longitudinal axis due to its chemical shift. The second 45°-pulse flips the fat magnetization back to the longitudinal axis, while the water magnetization is tipped further down to the transverse plane, having experienced 90° flip in total. Compared to spectral fat saturation, water excitation is quite insensitive to B1 field inhomogeneity, but has similar problems with B0 field inhomogeneity.
Fat/water separation
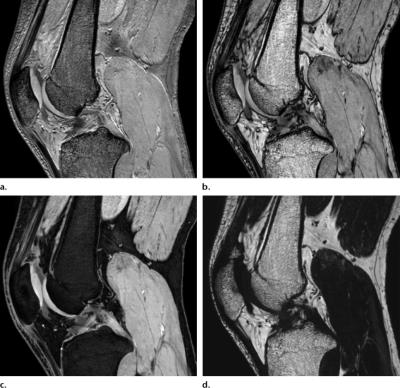
Dixon-type methods use chemical shift to separate water and fat signal into a water-only and a fat-only image. Since the water-only image is free from fat signal, fat/water separation works as a fat suppression technique. Unlike other methods, the fat suppression is effectively performed during post-processing and not during acquisition. The separation rather than suppression of signal has the advantage of providing an additional image of the fat signal5. The water and fat images can also be combined into a non-fat-suppressed image, sometimes called an in-phase image. Thus, the method offers images with and without fat suppression from the same acquisition (Fig. 1).
The method requires that at least two echoes (points) are acquired with different relative dephasing between water and fat. In the simplest case, originally described by Dixon5, the signal is sampled at two points with water and fat in-phase and opposed-phase, respectively. The water and fat images can then be obtained by simple addition and subtraction of these acquired images. Using more involved calculations, water/fat separation is possible using more flexible sampling schemes than the original in-phase/opposed-phase12-15, and any number of echoes/points16.
A major drawback with fat/water separation methods is the additional acquisition time associated with the acquisition of multiple echoes. This penalty is minimized using the two-point Dixon method, which is therefore preferred for fat suppression purposes. However, fat/water separation can also be used to quantify fat content on a pixel basis by calculating the fat signal fraction17. In such quantitative applications, the quantification accuracy benefits from sampling a larger number of points, typically about six. The accuracy of fat quantification is also improved by minimizing T1-weighting and incorporating T2*-decay and multiple fat resonances into the signal model18,19.
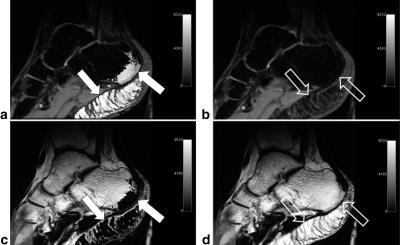
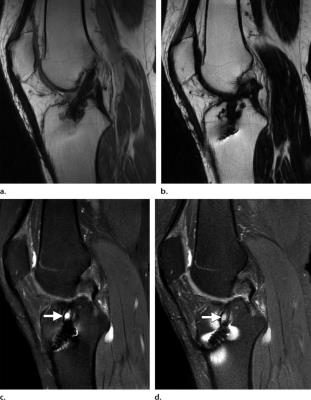
The most important improvement of Dixon’s original method is incorporation of B0-correction20,21. If this is omitted, fat/water separation will be sensitive to B0 inhomogeneity for the same reason as other methods based on chemical shift. On the contrary, if the B0-correction is successful, the fat/water separation will be fully insensitive to B0 inhomogeneity, which is a major advantage for these methods. Automatic, robust, and efficient B0-correction has proven a non-trivial and long-standing problem which has generated a large number of algorithms22. Methods based on graph cuts are notable among the most promising23,24. Local failure of the B0-correction results in so-called fat/water-swap artifacts. The appearance of these depends on the algorithm used, but they share the common feature that the water and fat signal switch places in parts of the image (Fig. 2).
It is important to be aware that fat/water separation is compatible with both gradient echo and spin echo based sequences5. In the gradient echo case, two-point Dixon is usually performed using a bipolar dual-echo readout gradient. In spin echo, the readout gradient can be shifted relative to the spin echo (asymmetric spin echo) to accumulate a phase difference between the water and fat magnetizations5. Various shifts can be used in different interleaves25, or a bipolar triple-echo acquisition can be performed centered at each spin echo26.
The separation of water and fat signal does not affect the contrast weighting, meaning that fat/water separated images may be PD-weighted, T1-weighted, T2-weighted, or T2*-weighted.
Results
An example use of fat/water separation in MSK is shown in Fig. 3. A PD-weighted Dixon acquisition provides similar image quality with similar acquisition time as a conventional PD-weighted image with spectral fat saturation, while additionally providing an image without fat suppression (water+fat) and a fat-only image. The fat image provides anatomical reference and information about fat content, and may therefore replace a conventional T1-weighted image for these purposes27.
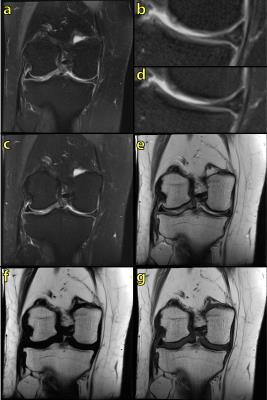
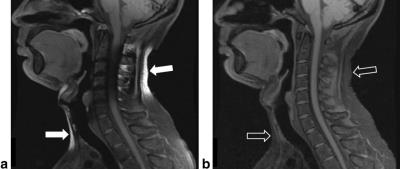
However, the major advantage of fat/water separation as a fat suppression method is its insensitivity to B0 inhomogeneity. This is not evident in Fig. 3, since the B0 inhomogeneity is quite benign in this case. At other anatomical locations, B0 inhomogeneity can be more severe. This includes off-center imaging (shoulder, elbow, wrist), as well as regions with large susceptibility induced field variation such as the neck (Fig. 4). Large B0 inhomogeneity may also be induced by metallic implants (Fig. 5). If fat suppression is desired near metallic implants, fat/water separation should be considered.
Discussion
There are many situations where an MSK examination can benefit of Dixon-type fat/water separation, due to its ability to provide fat suppression insensitive to B0 inhomogeneity and the possibility to obtain multiple images from a single acquisition, including images with and without fat suppression. In contrast enhanced examinations, fat/water separation can provide images both with and without fat suppression before and after contrast administration.
Compared to STIR, which also offers fat suppression insensitive to field inhomogeneity, fat/water separation is compatible with contrast enhancement, offers higher SNR, and is more flexible with respect to image contrast.
Since the required B0 correction relies on quite involved post-processing algorithms, it is important to be aware of fat/water swap-artifacts. While the exact appearance of these depends on the algorithm used, they are often easy to read through when identified.
The main disadvantage of fat/water imaging is the increased acquisition time, typically by a factor two for spin echo based two-point Dixon. However, the SNR also increases since more time is spent sampling the signal. This higher SNR creates capacity to accelerate by other means to compensate for the additional acquisition time. If signal averaging is used, the NSA can be decreased to trade the higher SNR for shorter acquisition time. Similarly, any phase oversampling can be reduced as long as no unwanted aliasing is introduced. The additional SNR may also enable more acceleration by means of partial Fourier and/or parallel imaging. On a protocol level, the total acquisition time can be shortened if the multiple images provided by a Dixon acquisition can replace several conventional images. In some situations, a Dixon fat-only image may alleviate the need for a T1-weighted image. A single Dixon acquisition is a good alternative to acquiring two images with and without spectral fat saturation.
In conclusion, fat/water separation enables fat suppression insensitive to field inhomogeneity, and should be considered when fat suppression is desired imaging off-center, using large fields of view, or at anatomical sites with large patient induced field inhomogeneity, or near metallic implants. The multiple images provided by a single Dixon acquisition may replace several conventional images, in particular images acquired both with and without spectral fat saturation.
Acknowledgements
The author would like to thank Magnus Tengvar, M.D., for helpful discussions, and Roberto Vargas for image acquisition.References
- Kaldoudi E, Williams SCR. Fat and water differentiation by nuclear magnetic resonance imaging. Concept Magnetic Res, 4(1):53–71, 1992.
- Delfaut EM, Beltran J, Johnson G, Rousseau J, Marchandise X, Cotton A. Fat suppression in MR imaging: techniques and pitfalls. Radiographics, 19(2):373–382, 1999.
- Bley TA, Wieben O, François CJ, Brittain JH, Reeder SB. Fat and water magnetic resonance imaging. J Magn Reson Imaging, 31(1):4–18, 2010.
- Earls JP, Krinsky GA. Abdominal and pelvic applications of opposed phase MR imaging. Am J Roentgenol, 169(4):1071–1077, 1997.
- Dixon WT. Simple proton spectroscopic imaging. Radiology, 153(1):189–194, 1984.
- Bydder GM, Young IR. MR Imaging: Clinical use of the inversion recovery sequence. J Comput Assist Tomogr, 9(4): 659–675, 1985.
- Haase A, Frahm J, Hänicke W, Matthaei D. 1H NMR chemical shift selective (CHESS) imaging. Phys Med Biol, 30(4):341–344, 1985.
- Oh C, Hilal S, Cho Z. Selective partial inversion recovery (SPIR) in steady state for selective saturation magnetic resonance imaging (MRI). In: Book of Abstracts: Seventh Annual Meeting of the Society of Magnetic Resonance in Medicine (Vol 2), page 1042, 1988.
- Foo TK, Sawyer AM, Faulkner WH, Mills DG. Inversion in the steady state: contrast optimization and reduced imaging time with fast three dimensional inversion-recovery-prepared GRE pulse sequences. Radiology, 191(1):85 –90, 1994.
- Meyer CH, Pauly JM, Macovskiand A, Nishimura DG. Simultaneous spatial and spectral selective excitation. Magn Reson Med, 15(2):287–304, 1990.
- Del Grande F et al. Fat-Suppression Techniques for 3-T MR Imaging of the Musculoskeletal System. Radiographics, 34:217–233, 2014.
- Xiang QS. Two-point water-fat imaging with partially-opposed-phase (POP) acquisition: An asymmetric Dixon method. Magn Reson Med, 56(3):572–584, 2006.
- Bydder M, Yokoo T, Yu H, Carl M, Reeder SB, Sirlin CB. Constraining the initial phase in water-fat separation. Magn Reson Imaging, 29(2):216–221, 2011.
- Berglund J, Ahlström H, Johansson L, Kullberg J. Two-point Dixon method with flexible echo times. Magn Reson Med 2011;65:994–1004.
- Eggers H, Brendel B, Duijndam A, Herigault G. Dual-echo Dixon imaging with flexible choice of echo times. Magnetic Resonance in Medicine, 65(1):96–107, 2011.
- An L, Xiang QS. Chemical shift imaging with spectrum modeling. Magn Reson Med 2001;46:126–130.
- Lee JK, Dixon WT, Ling D, Levitt RG, Murphy WA. Fatty infiltration of the liver: demonstration by proton spectroscopic imaging. Preliminary observations. Radiology, 153(1):195–201, 1984.
- Yu H, Shimakawa A, McKenzie CA, Brodsky E, Brittain JH, Reeder SB. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med 2008;60:1122–1134.
- Hines CDG et al. T1 independent, T2* corrected chemical shift based fat.water separation with multi-peak fat spectral modeling is an accurate and precise measure of hepatic steatosis. J Magn Reson Imaging, 33(4):873–881, 2011.
- Yeung HN, Kormos DW. Separation of true fat and water images by correcting magnetic field inhomogeneity in situ. Radiology 1986;159:783–786.
- Kim YS, Mun CW, Cho ZH. Chemical-shift imaging with large magnetic field inhomogeneity. Magn Reson Med, 4(5):452–460, 1987.
- Ma J. Dixon techniques for water and fat imaging. J Magn Reson Imaging, 28(3):543–558, 2008.
- Hernando D, Kellman P, Haldar JP, Liang Z-P. Robust water/fat separation in the presence of large field inhomogeneities using a graph cut algorithm. Magn Reson Med 2010;63:79–90.
- Berglund J, Skorpil M. Multi-scale graph cut algorithm for efficient water-fat separation. Magn Reson Med, 2016 [doi: 10.1002/mrm.26479].
- Hardy PA, Hinks RS, Tkach JA. Separation of fat and water in fast spin-echo MR imaging with the three-point Dixon technique. J Magn Reson Imaging, 5(2):181–185, 1995.
- Son JB et al. A flexible fast spin echo triple-echo Dixon technique. Magn Reson Med, 77: 1049–1057, 2017.
- Guerini H et al. Fat suppression with Dixon techniques in musculoskeletal magnetic resonance imaging: A pictorial review. Semin Musculoskelet Radiol 19(4):335–347, 2015.
Figures