Magnetic Nanoparticle Theranostics for Brain Tumors
1Neurosurgery, Icahn School of Medicine at Mount Sinai
Synopsis
Magnetic nanoparticle theranostics will be discussed as a novel method to target brain tumors with MRI guidance. Both antibody conjugated magnetic iron-oxide nanoparticles (IONPs) and free IONPs will be discussed. MRI-guided convection-enhanced delivery (CED) will be introduced as an optimal delivery method for magnetic nanoparticle theranostic targeting of brain tumors in multiple rodent glioma models. Preliminary feasibility and efficacy results of an antibody conjugated magnetic nanoparticle theranostic utilized in a canine spontaneous glioma trial will be shown. Future work involving a magnetic nanoparticle theranostic with trimodal functionality (targeted cell therapy, hyperthermia, and MR imaging) will be introduced.
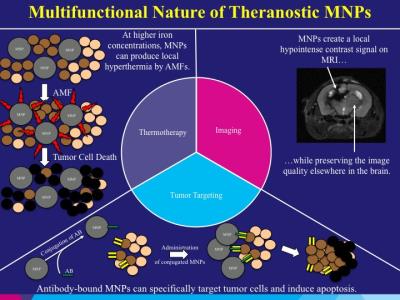
Magnetic Nanoparticle Theranostics for Brain Tumors: Nanotheranostics are currently being explored for use in the treatment of malignant brain tumors. Magnetic nanoparticles (MNPs) are an attractive theranostic due to their sensitive imaging qualities with magnetic resonance imaging (MRI) and their ability to target tumors. Malignant brain tumors remain a formidable cancer to treat. Glioblastoma (GBM) represents the most common primary malignant brain tumor that carries a poor prognosis for patients. GBM tumors are quite infiltrative into the surrounding normal brain permitting tumors to recur locally in the majority of patients despite all standard treatments.
MNPs have been designed to target GBM tumors both in preclinical work and human patient clinical trials. Magnetic iron oxide nanoparticles (IONPs) in the size range of 10-15 nm have unique magnetic properties, which generate significant transverse T2 relaxation time shortening and susceptibility effects resulting in strong T2 weighted contrast on MRI. Most IONPs are biodegradable and considered to have low toxicity. IONPs have been used in the clinical setting and proven to be safe for human use. Surface chemistries permit the functionalization and conjugation of ligands on MNPs for optimizing their delivery and targeting effect on brain tumors. Our group has focused on the conjugation of epidermal growth factor receptor (EGFR) antibodies (EGFRvIIIAb and cetuximab) to MNPs for therapeutic tumor targeting. EGFR, including the EGFRvIII deletion mutant, is overexpressed in the majority of GBM tumors and represents a major target for treatment of these tumors. Compared to EGFR antibodies alone, our data support the findings of increased binding by cetuximab-IONPs to EGFR and EGFRvIII-expressing GBM cells, including glioma stem-like cells (GSCs). Greater binding of cetuximab-IONPs and EGFR inhibition results in downstream EGFR cell signaling aberrations. We have also found greater intracellular presence of cetuximab-IONPs and greater translocation of EGFR into the cytoplasm, specifically the cytoskeletal fraction of cells. In combination, greater binding to EGFR, inhibition of EGFR, as well as internalization of the cetuximab-IONPs and EGFR trigger apoptosis in human EGFR-expressing GBM cells including GSCs. Radiosensitivity enhancement is also found with combination fractionated radiotherapy due to increased DNA double strands breaks (DSBs), as well as increased reactive oxygen species (ROS) formation after cetuximab-IONP treatment. The use of alternating magnetic fields (AMFs) can also provide a greater therapeutic effect by MNPs with the generation of local hyperthermia, known as thermotherapy. Thermotherapy of GBM may permit other adjuvant therapies such as radiotherapy and chemotherapy to be more efficacious. A few thermotherapy clinical trials with MNPs have been conducted in humans in recent years with positive results. In a study with patients diagnosed with primary GBM, external beam radiotherapy with adjuvant MHT was found to be safe, the MNPs created a localized hyperthermia effect, and local tumor control was observed. Post-mortem, MNPs and their aggregates were mainly found in macrophages within the tumor mass as well as in GBM cells. In a larger Phase II study involving patients with recurrent GBM, external beam radiotherapy with adjuvant MHT resulted in a significantly longer overall survival following diagnosis of first tumor recurrence. The median overall survival following primary tumor diagnosis was also greater. We will describe our initial results with generation of local hyperthermia with MNPs in a rodent model.
In order to overcome the blood brain barrier (BBB), we have used convection-enhanced delivery (CED) for robust delivery of MNPs within malignant brain tumors and the region of brain surrounding tumors where infiltrating cancer cells reside. CED permits distribution of molecules through the brain interstitial spaces by a pressure gradient applied through a catheter implanted in the brain. Direct delivery into the brain can provide higher concentrations of therapeutic agents in and around brain tumors while minimizing systemic toxic effects. CED of MNPs allows for the dispersion of nanoparticles within or adjacent to brain tumors and long retention after initial distribution. Shortening of tissue T2 relaxation time by IONPs provided contrast for MRI to follow the distribution of the nanoparticle complex. Our group has successfully performed intracranial CED of cetuximab-IONPs in a healthy canine model previously. Robust delivery of nanoparticles into the brain was achieved by CED and effective quantitative monitoring of distribution and dispersion of nanoparticles after CED was provided by MRI. No safety, intracranial or systemic toxicity issues were found after CED of cetuximab-IONPs. The aim of this pilot study was to evaluate the safety and potential efficacy of cetuximab-IONPs CED for treatment of spontaneously occurring gliomas in canines. Here we describe the current use of MNPs for targeted therapy of malignant brain tumors and future opportunities of these theranostic nanoparticles.
Acknowledgements
Alexandros Bouras, MD, Lab Manager (Hadjipanayis Laboratory), Mount Sinai Brain Tumor Nanotechnology Laboratory, Department of Neurosurgery, Tisch Cancer Institute at Mount Sinai, New York, NY.
Dominique Bozec, BS, Research Associate (Hadjipanayis Laboratory), Mount Sinai Brain Tumor Nanotechnology Laboratory, Department of Neurosurgery, Tisch Cancer Institute at Mount Sinai, New York, NY.
A. Courtenay Freeman, DVM, Veterinary Speciality Care, Mount Pleasant, SC.
Robert Ivkov, PhD, Department of Radiation Oncology and Molecular Radiation Sciences, Johns Hopkins University School of Medicine, Baltimore, MD.
Milota Kaluzova, PhD, Research Associate, Winship Cancer Institute of Emory University, Atlanta, GA.
Keon Mahmoudi, BS, Medical Student (Hadjipanayis Laboratory), Mount Sinai Brain Tumor Nanotechnology Laboratory, Department of Neurosurgery, Tisch Cancer Institute at Mount Sinai, New York, NY.
Simon R. Platt, BVM&S, MRCVS, Department of Small Animal Medicine and Surgery, College of Veterinary Medicine, University of Georgia, Athens, GA.
References
BOURAS A, KALUZOVA M, HADJIPANAYIS CG. 2015. Radiosensitivity enhancement of radioresistant glioblastoma by epidermal growth factor receptor antibody-conjugated iron-oxide nanoparticles. J Neurooncol, 124, 13-22.
HADJIPANAYIS, C. G., MACHAIDZE, R., KALUZOVA, M., WANG, L., SCHUETTE, A. J., CHEN, H., WU, X. & MAO, H. 2010. EGFRvIII Antibody–Conjugated Iron Oxide Nanoparticles for Magnetic Resonance Imaging–Guided Convection-Enhanced Delivery and Targeted Therapy of Glioblastoma. Cancer Research, 70, 6303-6312.
KALUZOVA, M., BOURAS, A., MACHAIDZE, R. & HADJIPANAYIS, C. G. 2015. Targeted therapy of glioblastoma stem-like cells and tumor non-stem cells using cetuximab-conjugated iron-oxide nanoparticles. Oncotarget, 6, 8788-806.
MAHMOUDI K, HADJIPANAYIS CG. 2014. The application of magnetic nanoparticles for the treatment of brain tumors. Front Chem, 2, 109.
MAIER-HAUFF, K., ULRICH, F., NESTLER, D., NIEHOFF, H., WUST, P., THIESEN, B., ORAWA, H., BUDACH, V. & JORDAN, A. 2011. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol, 103, 317-24.
PLATT, S., NDUOM, E., KENT, M., FREEMAN, C., MACHAIDZE, R., KALUZOVA, M., WANG, L., MAO, H. & HADJIPANAYIS, C. G. 2012. Canine Model of Convection-Enhanced Delivery of Cetuximab Conjugated Iron-Oxide Nanoparticles Monitored with Magnetic Resonance Imaging. Clinical neurosurgery, 59, 107-113.