Real-Time MRI Technology
1Centre for Cardiovascular Imaging, University College London, London, United Kingdom
Synopsis
Real-time MRI is the ability to continually acquire high temporal resolution data. Technological advances which have enabled high temporal and spatial resolution real-time MRI, include; i) hardware improvements; ii) efficient acquisition strategies (non-Cartesian trajectories and data undersampling); iii) reconstruction algorithms (parallel imaging, temporal encoding, Compressed Sensing), iv) rapid reconstruction (data reduction and parallelization on GPUs).
Highlights
- Hardware improvements; gradient systems and coils
- Acquisition strategy; Non-Cartesian trajectories and data undersampling
- Reconstruction techniques; Parallel imaging, Temporal encoding, and Compressed Sensing
- Reconstruction speed-ups; Data reduction and Graphics Processing Units- Real-time processing
Target audience
Participants interested in how real-time MR can be achieved;
- How we can speed up imaging
- Reconstruction algorithms
- Speeding up of reconstructions
- Real-time processing
Outcome/Objectives
- Understand how fast imaging can be achieved; efficient trajectories and data undersampling
- Understand reconstruction algorithms available; parallel imaging and compressed sensing
- Understood how fast visualization can be achieved; data reduction and GPUs
- Understand how real-time processing can be achieved and used
Real-time MRI Technology
MRI is inherently slow, making imaging of moving structures challenging.
Real-time MRI is the ability to continually acquire high temporal resolution data. Advances which have enabled high temporal and spatial resolution real-time MRI, include;
- Hardware improvements; gradient systems and coils
- Acquisition strategy; Non-Cartesian trajectories and data undersampling
- Reconstruction techniques; Parallel imaging, temporal encoding and Compressed Sensing
As well as fast acquisition, real-time imaging should ideally have:
- Rapid reconstruction and display of the resulting images
- Possible operator control to interact with the acquisition parameters
Real-time MRI is most commonly used for:
- Cardiovascular imaging (1)
- Speech imaging (2)
- Image guidance for interventional procedures (3)
- Joint movement (4)
Hardware improvements;
- Advanced in Gradients; decreased time to traverse k-space
- Advances in Coils; Maximising SNR and allowing maximum acceleration
Acquisition strategy;
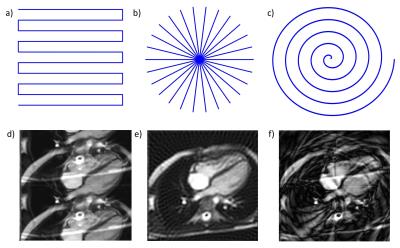
Cartesian acquisitions are slow as only a small amount of k-space if covered after each excitation. Alternative, efficient trajectories include;
- Echo Planar Imaging (EPI, figure 1a) (5); single shot or as a multi-shot acquisition
- Radials (figure 1b) (6); robust to undersampling
- Spirals (figure 1c) (7); efficient readout
Undersampling
Reducing the amount of k-space data collected results in spatial aliasing; (figure 1c-e).
Reconstruction techniques;
Reconstruction techniques that allows us to recover images from the undersampled k-space data, include;
* Parallel imaging; uses spatially independent information from multiple coils
- SENSE (Sensitivity Encoding) (8); works in image domain. Non-Cartesian SENSE requires use of iterative conjugate gradient algorithm (9)
- GRAPPA (10); works in k-space. Non-Cartesian GRAPPA uses independent kernels for different segments of space (11)
* Temporal encoding; alternating the missing lines in k-space in subsequent frames
- Keyhole imaging (12)
- TGRAPPA; temporal information used to calibrate weights (13)
- TSENSE; temporal information used to calculate coil sensitivity maps (14)
- Further exploited to improve the reconstruction, and enable higher accelerating to be achieved; k-t GRAPPA (15) and k-t SENSE (16)
* Compressed Sensing (17); exploits data sparseness and compressibility
- Temporal finite difference; real-time speech (18)
- Spatio-temporal sparsity (k-t SPARSE-SENSE); real-time cardiac cine (19)
Reconstruction Speed-ups;
Fast reconstruction is challenging due to the large data sets, and complexity of reconstruction algorithms.
*Data reduction; to reduce reconstruction times;
- Selection of most suitable coil subset (20)
- Array compression to combine channels (21)
*Graphics Processing Units (GPUs); massively parallel architecture to speed up algorithms (compared to CPUs; central processing units) that can be parallelized (22)
- Non-uniform FFT (23); up to x85
- Cartesian SENSE (24); up to ~x36
- Spiral SENSE (25); up to ~x15
- Compressed sensing (26); up to ~x27
Post-processing;
*Slice tracking;
- Exercise
- Intervention
*Segmentation;
- Vocal tract analysis (27)
- Flow calculation; Aortic segmentation (28)
- 3D kinematics of the knee (29)
Acknowledgements
Funding provided by Royal Society-EPSRC Dorothy Hodgkin Research FellowshipReferences
1. Sayin O, Saybasili H, Zviman MM, Griswold M, Halperin H, Seiberlich N, Herzka DA. Real-time free-breathing cardiac imaging with self-calibrated through-time radial GRAPPA. MRM 2017;77(1):250-264.
2. Lingala SG, Zhu Y, Lim Y, Toutios A, Ji Y, Lo W-C, Seiberlich N, Narayanan S, Nayak KS. Feasibility of through-time spiral generalized autocalibrating partial parallel acquisition for low latency accelerated real-time MRI of speech. MRM 2017.
3. Ozenne V, Toupin S, Bour P, de Senneville BD, Lepetit-Coiffé M, Boissenin M, Benois-Pineau J, Hansen MS, Inati SJ, Govari A, Jaïs P, Quesson B. Improved cardiac magnetic resonance thermometry and dosimetry for monitoring lesion formation during catheter ablation. MRM 2017;77(2):673-683.
4. Mazzoli V, Nederveen AJ, Oudeman J, Sprengers A, Nicolay K, Strijkers GJ, Verdonschot N. Water and fat separation in real-time MRI of joint movement with phase-sensitive bSSFP. MRM 2016.
5. Mansfield P. MULTI-PLANAR IMAGE-FORMATION USING NMR SPIN ECHOES. Journal of Physics C-Solid State Physics 1977;10(3):L55-L58.
6. Glover GH, Pauly JM. Projection Reconstruction Techniques for Reduction of Motion Effects in MRI. MRM 1992;28(2):275-289.
7. Meyer CH, Hu BS, Nishimura DG, Macovski A. Fast Spiral Coronary Artery Imaging. MRM 1992;28(2):202-213.
8. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. MRM 1999;42(5):952-962.
9. Pruessmann KP, Weiger Mk, Bornert P, Boesiger P. Advances in sensitivity encoding with arbitrary k-space trajectories. MRM 2001;46(4):638-651.
10. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). MRM 2002;47(6):1202-1210.
11. Griswold M, Heidemann R, Jakob P. Direct parallel imaging reconstruction of radially sampled data using GRAPPA with relative shifts. 2003; Toronto, Canada. p 2349.
12. Duerk JL, Lewin JS, Wu DH. Application of keyhole imaging to interventional MRI: A simulation study to predict sequence requirements. Journal of Magnetic Resonance Imaging 1996;6(6):918-924.
13. Breuer FA, Kellman P, Griswold MA, Jakob PM. Dynamic autocalibrated parallel imaging using temporal GRAPPA (TGRAPPA). MRM 2005;53(4):981-985.
14. Kellman P, Epstein FH, McVeigh ER. Adaptive sensitivity encoding incorporating temporal filtering (TSENSE). MRM 2001;45(5):846-852.
15. Huang F, Akao J, Vijayakumar S, Duensing GR, Limkeman M. k-t GRAPPA: A k-space implementation for dynamic MRI with high reduction factor. MRM 2005;54(5):1172-1184.
16. Tsao J, Boesiger P, Pruessmann KP. k-t BLAST and k-t SENSE: dynamic MRI with high frame rate exploiting spatiotemporal correlations. Magn Reson Med 2003;50(5):1031-1042.
17. Lustig M, Donoho D, Pauly JM. Sparse MRI: The application of compressed sensing for rapid MR imaging. MRM 2007;58(6):1182-1195.
18. Lingala SG, Zhu Y, Kim Y-C, Toutios A, Narayanan S, Nayak KS. A fast and flexible MRI system for the study of dynamic vocal tract shaping. MRM 2017;77(1):112-125.
19. Feng L, Srichai MB, Lim RP, Harrison A, King W, Adluru G, Dibella EVR, Sodickson DK, Otazo R, Kim D. Highly accelerated real-time cardiac cine MRI using k–t SPARSE-SENSE. MRM 2013;70(1):64-74.
20. Müller S, Umathum R, Speier P, Zühlsdorff S, Ley S, Semmler W, Bock M. Dynamic coil selection for real-time imaging in interventional MRI. MRM 2006;56(5):1156-1162.
21. Buehrer M, Pruessmann KP, Boesiger P, Kozerke S. Array compression for MRI with large coil arrays. MRM 2007;57(6):1131-1139.
22. Thomas S, Ti-chiun C, Peter S, Rudiger W. MR image reconstruction using the GPU. In: Michael JF, Jiang H, editors2006. SPIE. p 61423T.
23. Sorensen TS, Schaeffter T, Noe KO, Hansen MS. Accelerating the Nonequispaced Fast Fourier Transform on Commodity Graphics Hardware. Medical Imaging, IEEE Transactions on 2008;27(4):538-547.
24. Hansen MS, Atkinson D, Sorensen TS. Cartesian SENSE and k-t SENSE reconstruction using commodity graphics hardware. MRM 2008;59(3):463-468.
25. Kowalik GT, Steeden JA, Pandya B, Odille F, Atkinson D, Taylor A, Muthurangu V. Real-time flow with fast GPU reconstruction for continuous assessment of cardiac output. Journal of Magnetic Resonance Imaging 2012;36(6):1477-1482.
26. Smith DS, Gore JC, Yankeelov TE, Welch EB. Real-Time Compressive Sensing MRI Reconstruction Using GPU Computing and Split Bregman Methods. International Journal of Biomedical Imaging 2012;2012:6.
27. Silva S, Teixeira A. Unsupervised segmentation of the vocal tract from real-time MRI sequences. Computer Speech & Language 2015;33(1):25-46.
28. Mendonça GMQ, Carvalho JLA. Segmentation of aortic flow in real time magnetic resonance images. 2013 3-7 July 2013. p 6059-6062.
29. Lin C-C, Zhang S, Frahm J, Lu T-W, Hsu C-Y, Shih T-F. A slice-to-volume registration method based on real-time magnetic resonance imaging for measuring three-dimensional kinematics of the knee. Medical Physics 2013;40(10):102302.