How Does Gd Enter the Brain, When the BBB is Intact?
Synopsis
Both linear-type and macrocyclic-type gadolinium based contrast agents (GBCAs) enter the CSF and perivascular spaces even without BBB disruption and renal insufficiency. Intrathecally injected GBCAs distribute in the brain parenchyma when the BBB is intact. The glymphatic pathway, the waste clearance system of the brain, is key to the Gd deposition issue.
Target Audience
MR physicists, radiological technologists, neurologists, neurosurgeons, and all others involved with MR examinations using GBCAsOutcome/objectives
To increase understanding of the Gd deposition in the brain. To be able to use GBCAs appropriately. To be familiar with the concept of the glymphatic pathway, which may be important with regard to many neurodegenerative diseases.Introduction
Gadolinium deposition in the brain is receiving widespread attention from the general medical community. This phenomenon was first reported by Kanda, T. et al (1). After this initial report, numerous subsequent studies suggested that gadolinium deposition occurs in patients without renal insufficiency and without BBB disruption. The degree of deposition is more pronounced in patients that received multiple administrations of linear-type agents. Deposition sites are mainly the dentate nucleus, globus pallidus, and posterior thalamus. However, in extreme cases there may be an accumulation of gadolinium in the cerebral cortex along the central and calcarine sulci. These regions have a predilection for iron deposition. Therefore, gadolinium deposition in the brain might relate to iron metabolism. However, it is still unknown how gadolinium enters the brain parenchyma when the BBB is intact and renal function is normal.Methods and results
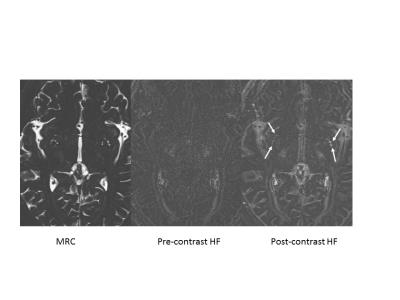
For the evaluation of inner ear endolymphatic hydrops, we routinely perform MR imaging at 4 hours after intravenous administration of a single dose of gadolinium based contrast agent (IV-SD-GBCA). To visualize very low concentrations of GBCAs in perilymph fluid, we use a heavily T2 weighted 3D-FLAIR sequence (3). During these examinations, we noticed that various fluid spaces were enhanced by the GBCA, such as the CSF, anterior eye segment, subarachnoid space of Meckel’s cave, and the fluid around the optic nerve (4). Based on the results, the blood-CSF barrier seems to be leakier than the BBB. Recently confirmed in animal models, the “Glymphatic system” is the waste clearance pathway of the brain (5). CSF flows through the periarterial / perivascular space (PVS) and enters the brain parenchyma through AQ4 channels. From there, the flow removes accumulated proteins like amyloid beta from the brain parenchyma through the perivenous PVS and into the CSF space. Multiple reports have indicated brain parenchymal enhancement after intrathecal administration of GBCAs (6, 7). Oner AY, et al. recently reported that patients who received a small amount of GBCA via intrathecal administration and had not received IV-GBCA showed increased signal in the dentate nucleus years after the intrathecal GBCA administration (8). We reported that the CSF and PVS are both enhanced by IV-SD-GBCA (both linear and macrocyclic agents) in human subjects without renal insufficiency or BBB disruption (Fig). (9). Jost, et al. reported that in an animal model, the CSF signal increased with IV administration of either a linear or macrocyclic agent (10). In a more recent study, intravenous administration of either linear or macrocyclic agents in rats caused an increase of gadolinium concentration in brain tissue samples at 24 hours after injection (11).Discussion
From these reports, we propose that intravenously administered GBCAs, either linear or macrocyclic agents, enter the CSF space first, and subsequently enter the PVS. The GBCAs would then enter the brain parenchyma. Only linear agents might be de-chelated, and bind with macromolecules such as transferrin, with gadolinium binding to macromolecule deposits primarily in iron-deposition predilection areas. The gadolinium bound to macromolecules might have a higher relaxivity than its original contrast agent state. Therefore, even with a very small amount of accumulation, a significant signal increase would occur. Or gadolinium might target ferroportin-rich area of neuronal tissue that are involved in active regulation of iron metabolism. The function of the glymphatic system is more activated during sleep and decreases with aging and traumatic brain injuries. It is also reported that brain radiation therapy and chemotherapy promote gadolinium deposition in the brain. We need further research on how to minimize gadolinium deposition and how much deposition is risky for brain tissue or brain function. We need to conduct both histological analyses in animals ex vivo as well as behavior analyses in vivo to better understand the impact of gadolinium deposition on brain function and health. In humans, functional brain imaging modalities such as FDG-PET or perfusion assessment by SPECT might be valuable to objectively evaluate functional alterations associated with gadolinium deposition.Conclusion
Until we can further elucidate the mystery of gadolinium deposition in the brain, we, as medical professionals, should try to reduce potential risks of such deposition by using GBCAs wisely.Acknowledgements
No acknowledgement found.References
(1) Kanda T, Ishii K, Kawaguchi H, Kitajima K, Takenaka D. High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology. 2014 Mar;270(3):834-41.
(2) Khant ZA, Hirai T, Kadota Y, Masuda R, Yano T, Azuma M, Suzuki Y, Tashiro K.T(1) Shortening in the Cerebral Cortex after Multiple Administrations ofGadolinium-based Contrast Agents. Magn Reson Med Sci. 2016 Oct 11. [Epub ahead ofprint]
(3) Naganawa S, Nakashima T. Visualization of endolymphatic hydrops with MRimaging in patients with Ménière's disease and related pathologies: currentstatus of its methods and clinical significance. Jpn J Radiol. 2014Apr;32(4):191-204.
(4) Naganawa S, Suzuki K, Yamazaki M, Sakurai Y. Serial scans in healthyvolunteers following intravenous administration of gadoteridol: time course ofcontrast enhancement in various cranial fluid spaces. Magn Reson Med Sci.2014;13(1):7-13.
(5) Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013 Jun28;340(6140):1529-30.
(6) Eide PK, Ringstad G. MRI with intrathecal MRI gadolinium contrast mediumadministration: a possible method to assess glymphatic function in human brain.Acta Radiol Open. 2015 Nov 17;4(11):2058460115609635. doi:10.1177/2058460115609635.
(7) Kapoor R, Liu J, Devasenapathy A, Gordin V. Gadolinium encephalopathy after intrathecal gadolinium injection. Pain Physician. 2010 Sep-Oct;13(5):E321-6.
(8) Öner AY, Barutcu B, Aykol S, Tali ET. Intrathecal Contrast-Enhanced MagneticResonance Imaging-Related Brain Signal Changes: Residual Gadolinium Deposition?Invest Radiol. 2016 Oct 13. [Epub ahead of print]
(9) Naganawa S, Nakane T, Kawai H, Taoka T. Gd-based Contrast Enhancement of thePerivascular Spaces in the Basal Ganglia. Magn Reson Med Sci. 2016 Jul 12. [Epub ahead of print]
(10) Jost G, Lenhard DC, Sieber MA, Lohrke J, Frenzel T, Pietsch H. Signal Increaseon Unenhanced T1-Weighted Images in the Rat Brain After Repeated, Extended Doses of Gadolinium-Based Contrast Agents: Comparison of Linear and Macrocyclic Agents.Invest Radiol. 2016 Feb;51(2):83-9.
(11) Jost G, Frenzel T, Lohrke J, Lenhard DC, Naganawa S, Pietsch H. Penetration and distribution of gadolinium-based contrast agents into the cerebrospinal fluid in healthy rats: a potential pathway of entry into the brain tissue. Eur Radiol. 2016 Nov 10. [Epub ahead of print]
Figures