Cardiac Devices: Safety Aspects & Challenges in Cardiac MR
Synopsis
This lecture will describe some of the safety and image artifact issues related to performing MRI in patients with an implantable device (e.g., pacemaker, ICD, CRT).
Target audience
Basic science and clinical imagers involved with cardiovascular MRI, particularly those with interests in heart failure and cardiac rhythm disorders.Objectives: Upon completion of this educational presentation, the participants should be able to:
- Understand some of the safety aspects for performing MRI safely in patients with an implantable defibrillator
- Understand some of the challenges for obtaining high image quality from patients with an implantable defibrillator
Purpose/Introduction:
Heart failure (HF) is a major healthcare problem in the United States (1) and worldwide. Both echocardiography and MRI play an important role in diagnosis, prognosis and risk stratification of patients with HF (2). MRI is arguably more versatile and capable of a comprehensive evaluation, including function of both left and right ventricles, perfusion, edema, and scar/fibrosis. Despite these advantages, MRI’s utility may be limited in many HF patients with left ventricular ejection ≤ 35% who also have a prophylactic implantable defibrillator. One could argue that these HF patients tend to be sicker and thus are the ones who would derive more benefit from MRI, since they are more likely to have structural heart disease that could be characterized with MRI. Despite the fact that MRI can be performed safely in most patients with a defibrillator at 1.5T (3-6), many patients who would derive benefit from MRI do not undergo MRI, largely due to image artifacts arising from the transformer of a device (e.g., pacemaker, implantable cardioverter defibrillator [ICD], cardiac resynchronization therapy implantable cardioverter defibrillator [CRT-D]).
Clinical Indications:
It is estimated that approximately 50% of patients with a defibrillator will be indicated for a clinical MRI exam during the lifetime of the device. Indications for MRI in patients with an implantable device: brain (40%), spine (22%), heart (16%), abdomen or pelvis (13%), and extremity (9%).Safety Concerns:
- Force and torque: The amount of ferromagnetic materials on a device is minimal, thus this is not considered to be a safety issue at 1.5T (7, 8).
- Device Functionality: Changes in device settings (e.g., reed-switch) may occur due to static magnetic field (B0), leading to asynchronous pacing in pacemakers and inhibition of shock delivery in ICDs. These need to be monitored carefully (4).
- Gradient Fields: Switching of magnetic field gradient could in theory induce currents to produce direct stimulation of the heart (9).
- Radiofrequency Fields: RF induced intracardiac lead tip heating has been demonstrated in vitro (10) and in vivo (11).
- These safety concerns can be mitigated – but not eliminated - by following an established device protocol at 1.5T (5, 6).
Image Artifact Concerns:
Both intracardiac leads and the transformer of a device act as sources of image artifacts. Intracardiac leads typically appear as signal void, but for the most part produce benign image artifacts. The transformer, on the other hand, causes significant center frequency shift (~kHz range) and generates significant image artifacts and signal dephasing.
- Standard cardiac pulse sequences using b-SSFP readout are sensitive to off-resonance effects, so they do not work in patients with a device.
- FLASH based sequences are able to suppress image artifacts, but two factors need to be considered, including geometric distortion and dephasing.
- Spin-echo based pulse sequences are able to suppress image artifacts and achieve insensitivity to dephasing, but one must be careful to use low RF deposition.
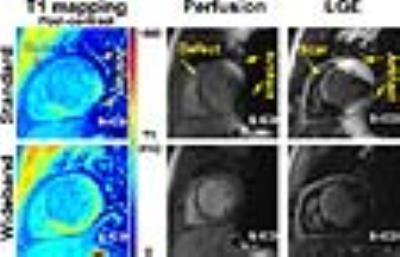
- Wideband cardiac pulse sequences have been introduced recently to suppress image artifacts for these applications: LGE (12, 13), T1 mapping (14, 15), and perfusion (16).
Results:
Figure 1 shows representative T1 maps, perfusion, and LGE images of a patient with a subcutaneous ICD (S-ICD), which is considerably larger than both ICD and CRT-D. Compared with standard acquisitions, wideband acquisitions suppressed image artifacts induced by S-ICD.Discussion:
As more academic centers become comfortable with performing cardiovascular MRI safely in patients with an implantable defibrillator, more investigations are needed to improve image quality and identify clinical utility in HF and cardiac rhythm disorders.Acknowledgements
No acknowledgement found.References
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123(8):933-44.
- Slaughter RE, Mottram PM. What Should Be the Principle Imaging Test in Heart Failure—CMR or Echocardiography? JACC: Cardiovascular Imaging. 2010;3(7):776-82.
- Nazarian S, Halperin HR. How to perform magnetic resonance imaging on patients with implantable cardiac arrhythmia devices. Heart Rhythm. 2009;6(1):138-43.
- Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Ann Intern Med. 2011;155(7):415-24.
- Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114(12):1277-84.
- Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114(12):1285-92.
- Schmiedel A, Hackenbroch M, Yang A, et al. [Magnetic resonance imaging of the brain in patients with cardiac pacemakers. Experimental and clinical investigations at 1.5 Tesla]. Rofo. 2005;177(5):731-44.
- Luechinger R, Duru F, Scheidegger MB, et al. Force and torque effects of a 1.5-Tesla MRI scanner on cardiac pacemakers and ICDs. Pacing Clin Electrophysiol. 2001;24(2):199-205.
- Levine GN, Gomes AS, Arai AE, et al. Safety of magnetic resonance imaging in patients with cardiovascular devices: an American Heart Association scientific statement from the Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Council on Cardiovascular Radiology and Intervention: endorsed by the American College of Cardiology Foundation, the North American Society for Cardiac Imaging, and the Society for Cardiovascular Magnetic Resonance. Circulation. 2007;116(24):2878-91.
- Achenbach S, Moshage W, Diem B, et al. Effects of magnetic resonance imaging on cardiac pacemakers and electrodes. Am Heart J. 1997;134(3):467-73.
- Luechinger R, Zeijlemaker VA, Pedersen EM, et al. In vivo heating of pacemaker leads during magnetic resonance imaging. Eur Heart J. 2005;26(4):376-83; discussion 25-7.
- Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270(1):269-74.
- Ranjan R, McGann CJ, Jeong EK, et al. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace. 2015;17(3):483-8.
- Hong K, Jeong EK, Kim D. Wideband arrhythmia-insensitive-rapid (AIR) cardiac T1 mapping pulse sequence for suppressing image artifacts induced by ICD. In: Proceedings of the 23rd Annual Meeting of ISMRM, Toronto, Ontario, Canada 2015. Abstract No. 6747.
- Shao J, Rashid S, Renella P, et al. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magn Reson Med. 2016.
- Kim D, Lee D, Wilcox J, et al. Wideband cardiovascular MRI for imaging patients with intracardiac device implantation. 103rd Annual Meeting at RSNA. Abstract No. 16018349.