Parallel Imaging
1Case Western Reserve University
Synopsis
This review of parallel imaging techniques will focus on learning basic principles and its clinical use. Specifically, we will discuss how data are accelerated, and the resulting aliasing artifacts that occur. We will explore how coil sensitivities and parallel imaging reconstruction methods can be used to reconstruct undersampled data. Lastly, we will review clinical applications of parallel imaging.
Target Audience
Clinicians interested in learning basic principles of parallel imaging and its clinical use.Objectives
- Provide an overview of accelerated data acquisition and its resulting aliasing artifacts
- Understand how coil sensitivities can be used to reconstruct undersampled data
- Review clinical applications of parallel imaging
Acquisition of MR images is relatively slow, and the resulting long exam times lead to increased costs and are a large source of patient discomfort. Additionally, faster imaging speed is important in several clinical applications, such as reducing breath-holds in cardiac and abdominal imaging exams. In MRI data acquisition, these long scan times occur because there is an inherent link between the scan duration, spatial resolution, field-of-view, and signal-to-noise ratio (SNR). A majority of MRI data are acquired in a time-consuming line-by-line fashion using frequency and phase encoding, and the total number of lines is determined by the field-of-view, spatial resolution, and how many averages of each line are acquired (to improve SNR). Therefore, in order to achieve the clinically desirable high spatial resolution, high spatial coverage, and high SNR images, imaging times become long.
Parallel imaging reconstructions were introduced in the 1990s and early 2000s (1–3), and have since become a frequently used method for reducing clinical scan times. These techniques rely on the use of a multi-channel receive array to acquire images. Each coil in the array simultaneously acquires the MRI signal and has a different, spatially-localized sensitivity, which provides additional spatial encoding information. By properly using the spatial encoding from the coil array, the amount of time-consuming phase encoding during data acquisition can be reduced.
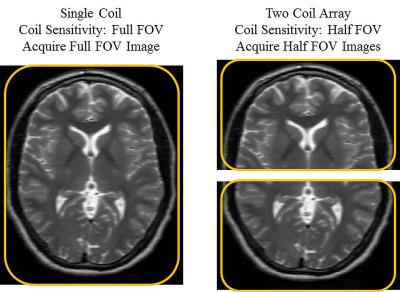
The basic concept of parallel imaging is described using an idealized example in the figure below. Without parallel imaging, the prescribed field-of-view, resolution and the repetition time determines the total imaging time of the acquisition. On the left, an image of the brain is acquired with a single coil. In this single coil image, parallel imaging cannot be applied because there is no additional spatial encoding from multiple coils. On the right, a two coil array is used to acquire this same image of the brain. When MRI data are acquired using multiple coils with spatially varying sensitivity, the images will be weighted (multiplied) by the coil sensitivity. The coil sensitivity for the first coil covers exactly the anterior half of the brain, and the coil sensitivity for the second coil covers exactly the posterior half of the brain. Thus, in this ideal case, the image acquired with each coil would be an image of the corresponding half of the brain with the other half of the image equaling zero. This additional spatial information from the coils is used to reduce the amount of phase encoding by halving the field-of-view, and will result in halving the total imaging time. The two half field-of-view images are acquired simultaneously with the two coils (shown right), and a final image can be reconstructed by combining these images. Although this example is not realizable in practice, it describes how multi-coil arrays can be used to acquire less data and to accelerate MR image acquisition.
This review of parallel imaging concepts will describe how these methods are employed in clinical use. In parallel imaging acquisitions, data are accelerated by skipping k-space lines in the phase encoding direction. This increased distance between k-space lines reduces the image field-of-view, which results in aliasing artifacts in the image domain. Parallel imaging techniques use information about the coil sensitivities to reconstruct an unaliased image from the undersampled data. Many successful parallel imaging reconstruction methods have been proposed, but this review will focus on two frequently used parallel imaging techniques: SENSE (Sensitivity Encoding) (2) and GRAPPA (Generalized Autocalibrating Partially Parallel Acquisition) (3). SENSE is applied in the image domain to unfold aliasing artifacts caused by the undersampling. GRAPPA is applied in k-space to estimate missing k-space lines. Regardless of the reconstruction method employed, all parallel imaging methods have several similarities (4–6):
- Data are acquired using an array of multiple independent coils, and each coil has a different spatial sensitivity over the field-of-view.
- Additional knowledge about the coil sensitivities is required for the image reconstruction.
- Undersampling the data by a factor R (to reduce imaging time by a factor R) results in a reduction of image SNR by a factor of at least √R.
Parallel imaging is an important tool to reduce imaging time with MRI. This review will introduce technical details of parallel imaging, including: data acquisition with coil arrays, data undersampling and aliasing, and parallel imaging reconstruction techniques. It will also summarize the clinical implementation of parallel imaging. The improvement in imaging speed from parallel imaging can be applied in several ways to clinical MR imaging. It can be used to shorten total scan time (as mentioned above), or it can be used to achieve higher spatial resolutions or larger field-of-views for the same scan time. Additionally, parallel imaging has been used in some imaging sequences (fast gradient echo, fast spin echo, echo planar imaging) to reduce imaging artifacts. This review will summarize this range of clinical applications of parallel imaging.
Acknowledgements
No acknowledgement found.References
1. Sodickson DK, Manning WJ. Simultaneous acquisition of spatial harmonics (SMASH): fast imaging with radiofrequency coil arrays. Magn. Reson. Med. 1997;38(4):591–603.
2. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: sensitivity encoding for fast MRI. Magn. Reson. Med. 1999;42(5):952–62.
3. Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, Wang J, Kiefer B, Haase A. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn. Reson. Med. 2002;47(6):1202–1210.
4. Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J. Magn. Reson. Imaging 2012;36(1):55–72.
5. Blaimer M, Breuer F, Mueller M, Heidemann RM, Griswold M a, Jakob PM. SMASH, SENSE, PILS, GRAPPA: how to choose the optimal method. Top. Magn. Reson. Imaging 2004;15(4):223–36.
6. Larkman DJ, Nunes RG. Parallel magnetic resonance imaging. Phys. Med. Biol. 2007;52(7):R15–55.