Microstural Investigation Using Diffusion Tensor Imaging
1Centre for the Developing Brain, King's College London, London
Synopsis
Objectives
- This talk will focus on brain development in infants born preterm
- Discuss differences in diffusion MRI measures in white- and grey-matter between preterm infants compared to term controls
- Assess relationship between dMRI measures and relevant perinatal clinical factors
- Discuss relationship between dMRI measures at term equivalent age and subsequent neurodevelopmental outcome
Background
Infants who are born preterm have a high prevalence of motor, cognitive and behavioral deficits which are evident in childhood1,2 and an increased risk of developing psychiatric disorders in adulthood.3 By studying brain growth and connectivity, magnetic resonance imaging (MRI) has been used extensively to improve our understanding of the neural substrate underlying neurodevelopmental impairments in this population. By term-equivalent age, the preterm brain is clearly different from that of a healthy term-born infant. Compared to their term-born peers, preterm infants display significantly lower fractional anisotropy (FA) throughout the white matter on diffusion MRI (dMRI). 4,5 These diffusion changes are part of more global differences in brain development following premature delivery including reductions in regional brain volume6,7 and altered cortical development.8,9Summary of Recent Studies
White matter development
Using high angular resolution multi-shell dMRI to assess connectivity between brain regions and connectivity metrics obtained from the diffusion tensor (fractional anisotropy, FA) and NODDI model characteristics (neurite density index, NDI), we assessed regional differences in brain maturation from 25 weeks gestation to term equivalent age.10 Brain development prior to term equivalent age is characterised by an increase in FA and NDI. Connections to and from deep grey matter showed most rapid developmental changes during this period while intra-frontal, frontal to cingulate, frontal to caudate and inter-hemispheric connections matured more slowly. This maturational gradient is consistent with the regional heterogeneity observed histologically with myelination progressing from inferior to superior and from posterior to anterior.11,12
Relationship with Perinatal Risk Factors
The preterm brain is susceptible to injury from ischaemic, haemorrhagic, inflammatory and infective insult: immature oligodendrocytes are particularly sensitive to injury, as are subplate neurons which form synapses with thalamo-cortical afferents in the transient subplate layer that are essential for normal cortical development. These vulnerabilities may help explain the abnormalities in brain growth and development observed using quantitative MR imaging in infants and children who were born preterm. We assessed the relationship between perinatal risk factors and white matter development in a large group of preterm infants (n = 491) at term equivalent age.13 Risk factors for diffuse white matter injury were lower GA at birth (Figure 1), fetal growth restriction, increased number of days requiring ventilation and parenteral nutrition, necrotizing enterocolitis and male sex. Exposure to multiple risk factors exacerbated white matter injury, supporting the multiple hit hypothesis for the variation in brain development observed following preterm birth.
Development of the Cerebral Cortex
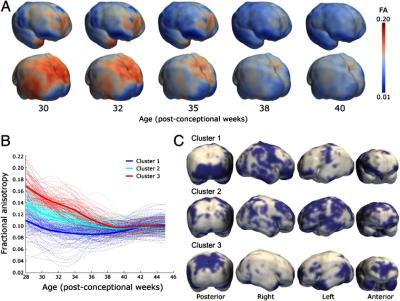
In addition to studying white matter, cortical maturation can be assessed using dMRI. Prior to 32 weeks gestation age, the cortex is dominated by perpendicular radial glia and apical dendrites, resulting in nonzero anisotropy,14 and is accompanied by decreases in both mean diffusivity and FA due to increasing cellular density, complexity, neurite overgrowth, and maturing dendritic cytoarchitecture.14-15 Cortical FA declines up to around 38 weeks gestational age, with higher initial values and rate of change in the frontal and temporal poles, and parietal cortex, and lower initial values in the perirolandic, and medial occipital cortices, suggesting that elongation and branching of dendrites orthogonal to cortical columns occurs later in the association cortex (Figure 2).15 Cortical development, as assessed by d-MRI, is impaired in preterm infants who have slower postnatal growth.16 Moreover, the rate of microstructural maturation correlates not only with local cortical growth but also importantly with neurodevelopmental performance at 2 years of age.15
Conclusion
Diffusion MRI offers the opportunity to assess preterm brain development in vivo. A number of studies have highlighted altered white and grey matter development in infants born preterm. This altered development is associated with exposure to perinatal risk factors and related to subsequent neurodevelopmental outcome. As such, dMRI may provide a biomarker for studies exploring mechanisms of white and grey matter injury and may expedite the assessment of efficacy of early interventions in this high-risk group of infants.Acknowledgements
I would like to thank the families who took part in this research and all the staff at the Centre for the Developing Brain, King's College London.References
- Bhutta et al. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA, 288 (2002), pp. 728–73.
- Saigal & Doyle. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet, 371 (2008), pp. 261–269.
- Nosarti et al. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain, 131 (2008), pp. 205–217.
- Anjari et al. Diffusion tensor imaging with tract-based spatial statistics reveals local white matter abnormalities in preterm infants. Neuroimage 2007;35(3):1021–7.
- Huppi et al. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res. 1998 Oct;44(4):584-90.
- Ball et al., The effect of preterm birth on thalamic and cortical development. Cereb Cortex, 2012. 22(5): p. 1016-24.
- Cheong et al. Brain Volumes at Term-Equivalent Age Are Associated with 2-Year Neurodevelopment in Moderate and Late Preterm Children. J Pediatr. 2016 Jul;174:91-97.
- Kapellou et al. Abnormal cortical development after premature birth shown by altered allometric scaling of brain growth. PLoS Med. 2006 Aug;3(8):e265.
- Dubois et al. Primary cortical folding in the human newborn: an early marker of later functional development. Brain. 2008;131:2028-41
- Batalle et al. Early development of structural networks and the impact of prematurity on brain connectivity. Neuroimage. 2017 Jan 30;149:379-392.
- Gilles, F., Shankle, W., Dooling, E., 1983. Myelinated tracts: growth patterns. The Developing Human Brain: Growth and Epidemiologic Neuropathology. Boston, John Wright, 117–183.
- P.I. Yakovlev, A.R. LeCours The myelogenetic cycles of regional maturation of the brain. Minkowski (Ed.), Regional Development Of The Brain In Early Life, Blackwell, Oxford (1967), pp. 3–70
- Barnett et al. Exploring the multiple-hit hypothesis of preterm white matter damage using diffusion MRI. Submitted.
- McKinstry et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002;59(6):824–33.
- Ball Get al. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci U S A 2013;110(23):9541–6.
- Vinall et al. Slower postnatal growth is associated with delayed cerebral cortical maturation in preterm newborns. Sci Transl Med 2013;5(168):168ra8.
Figures