MR US Fusion Guided Intervention for Pain Syndromes
Synopsis
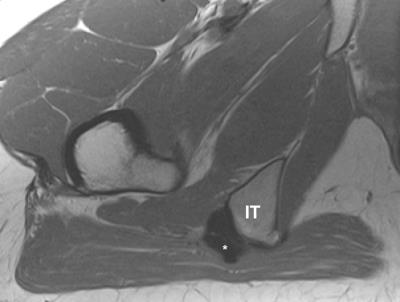
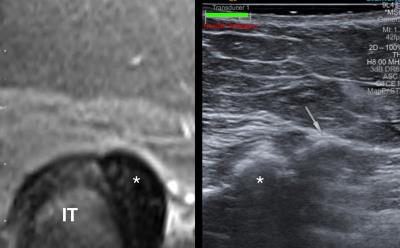
Ultrasound (US) systems equipped with position sensors can acquire three-dimensional spatial data allowing registration with previously acquired magnetic resonance (MR) imaging for fused real-time sonographic imaging. Co-registration and fusion of alignment involves sequential algorithmic transformations minimizing the error between the output and target image. An electromagnetic field generator is used to track transducer orientation to simultaneously map real-time US with corresponding anatomy on pre-acquired MR. The potential utility of this technology in the treatment of various pain syndromes in particular certain joint, tendon and perineural therapies will be described.
Acknowledgements
No acknowledgement found.References
1. Roche, A, Pennec, X, Malandain, G., Ayache, N. Rigid registration of 3D ultrasound with MR images: a new approach combining intensity and gradient information. IEEE Transactions on Medical Imaging. 2001; 20: 1038–1049.
2. Rohlfing, T, Maurer, JCR. Modeling liver motion and deformation during the respiratory cycle using intensity-based nonrigid registration of gated MR images. Medical Physics. 2004; 31: 427–432.
3. Wein W, Brunke S, Khamene A, Callstrom MR, Navab N. Automatic CT-ultrasound registration for diagnostic imaging and image-guided intervention. Med Image Anal. 2008;12(5):577-85.
4. Crocetti L, Lencioni R. DeBeni S, See T, Pina, C, Bartolozzi, C. 2008. Targeting liver lesions for radiofrequency ablation: an experimental feasibility study using a CT-US fusion imaging system. Investigative Radiology. 2008; 43: 33–39.
5. Lee MW. Fusion imaging of real-time ultrasonography with CT or MRI for hepatic intervention. Ultrasonography. 2014; 33(4):227-39.
6. Costa DN, Pedrosa I, Donato F Jr, Roehrborn CG, Rofsky NM. MR Imaging-Transrectal US Fusion for Targeted Prostate Biopsies: Implications for Diagnosis and Clinical Management. Radiographics. 2015; 35: 696-708.
7. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL, Linehan WM, Merino MJ, Simon RM, Choyke PL, Wood BJ, Pinto PA. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA. 2015;313(4):390-7.
8. Pons EP, Azcón FM, Casas MC, Meca SM, Espona JL. Real-time MRI navigated US: role in diagnosis and guided biopsy of incidental breast lesions and axillary lymph nodes detected on breast MRI but not on second look US. Eur J Radiol. 2014;83(6): 942-50.
9. Klauser AS, De Zordo T, Feuchtner GM, Djedovic G, Weiler RB, Faschingbauer R, Schirmer M, Moriggl B. Fusion of real-time US with CT images to guide sacroiliac joint injection in vitro and in vivo. Radiology. 2010; 256(2):547-53.
10. Zacchino M, Almolla J, Canepari E, Merico V, Calliada F. Use of ultrasound-magnetic resonance image fusion to guide sacroiliac joint injections: a preliminary assessment. J Ultrasound. 2013; 16(3):111-8.
11. Hu Z, Zhu J, Liu F, Wang N, Xue Q. Feasibility of US-CT image fusion to identify the sources of abnormal vascularization in posterior sacroiliac joints of ankylosing spondylitis patients. Sci Rep. 2015; 16; 5:18356
12. Wong-On M, Til-Pérez L, Balius R. Evaluation of MRI-US Fusion Technology in Sports-Related Musculoskeletal Injuries. Adv Ther. 2015; 32(6):580-94
13. Khalil JG, Mott MP, Parsons TW 3rd, Banka TR, van Holsbeeck M. 2011 Mid-America Orthopaedic Association Dallas B. Phemister Physician in Training Award: Can musculoskeletal tumors be diagnosed with ultrasound fusion-guided biopsy? Clin Orthop Relat Res. 2012; 470(8):2280-7.
14. Burke CJ, Bencardino J, Adler R. The Potential Use of Ultrasound-Magnetic Resonance Imaging Fusion Applications in Musculoskeletal Intervention. J Ultrasound Med. 2017; 36(1):217-224.
Figures