5664
An Artificial Neural Network Framework for Early Prediction of Cognitive Deficits in Very Preterm Infants1Pediatric Neuroimaging Research Consortium, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States, 2Perinatal Institute, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, United States
Synopsis
Annually, approximately 22,000 very preterm
infants (i.e. ≤32 weeks
gestational age) in the United States develop cognitive deficits. Infant brains are
highly malleable, making it especially important to identify those at highest
risk as early as possible to allow effective early interventions. Research
supports the notion that cognitive deficits may result from a
disturbance/breakdown in the connectome. We
propose to develop a robust artificial neural network framework that can analyze
integrated structural and functional brain connectome data obtained at term
corrected age to predict long-term cognitive outcomes in very preterm infants.
PURPOSE
Prematurely born infants exhibit a several fold increased risk for neurodevelopmental impairments.1 Annually, approximately 22,000 very preterm infants (i.e. ≤32 weeks gestational age) in the United States develop cognitive deficits.2 Infant brains are highly malleable, making it especially to identify those at highest risk as early as possible to allow effective early interventions. Research supports the notion that cognitive deficits may result from a disturbance/breakdown in the connectome. There is a growing interest in applying machine learning based on connectome data3 to predict neurological deficits, but its use in preterm populations is very limited.4 In this work, we propose to develop a robust artificial neural network framework (ANN) that can analyze integrated structural and functional brain connectome data obtained at term corrected age (CA) to make predictions about cognitive outcomes in very preterm infants.METHODS
The study population was derived from an imaging cohort of very preterm infants cared for in the neonatal ICU of Nationwide Children’s Hospital. MR imaging specifications were: 3T GE HDx scanner with 8-channel infant head coil; T2w – TR/TE 11000/185 ms, FA 90°, resolution 0.35 x 0.35 x 2 mm; dMRI – TR/TE 10000/87 ms, FA 90, resolution 2 x 2 x 2.4 mm, 30 non-collinear diffusion weighted directions, b-value 800 sec/mm2; functional connectivity MRI (fcMRI) – EPI TR/TE 3000/35 ms, FA 90°, resolution 2.8 x 2.8 x 3 mm. Twenty-three infants have now reached 2 years CA and completed their standardized Bayley Scales of Infant and Toddler Development-III test (Bayley-III). The Bayley-III normative cognitive scores are on a scale of 50 to 150, with a mean of 100 and standard deviation (SD) of 15. We grouped our cohort using a cut-off of 85 (1 SD below mean) in those at high vs. low risk for cognitive deficits (i.e. two classes). Both structural and functional connectomes for each of these infants were constructed.5,6 To address the challenges of relatively small and imbalanced (the number of preterm infants with low cognitive scores is relatively small) training set, we adopted an Adaptive Synthetic Sampling Approach for Imbalanced Learning (ADASYN)7 to augment and balance the training set. Training subjects were categorized into 3 groups per their cognitive scores (50-85, 86-127 and 128-150). We ran ADASYN repeatedly to generate synthetic samples from the group with the fewest total number of real and synthetic samples, until the number of training samples was augmented by a factor of 32. The synthetic data were only used for the model training, and not for the testing. This data augmentation strategy prevents model overfitting. We extracted the most representative high-level features using a stacked sparse autoencoder (SSAE) model.8 Based on the high-level connectome features, we predicted the risk of cognitive deficits using support vector machine (SVM).9 We also conducted the prediction using conventional multivariable statistical analyses for comparison. Specifically, we employed principle components analysis (PCA)10 to extract high-level connectome features, and implemented Generalized Linear Model (GLM)11 using PCA-based features to predict cognitive deficits. The models were tested on 1) structural, 2) functional and 3) integrated structural and functional brain connectome features. Leave-one-out cross-validation strategy was adopted to train and test the models. Reported results include classifier accuracy, sensitivity and specificity.RESULTS
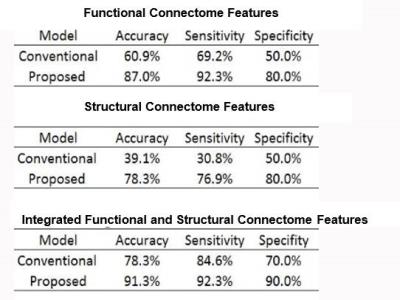
As shown in Table 1, integration of structural and functional connectome features produced superior performance in prediction of cognitive deficits than use of structural or functional connectome independently. Using integrated brain connectome features, we accurately classified 91.3% of very preterm infants at high risk of cognitive deficits with 92.3% sensitivity and 90% specificity. Our proposed ANN framework improved prediction accuracy by 13% compared to the conventional multivariable model; the improvements in sensitivity and specificity were 7.7% and 20%, respectively.DISCUSSION AND CONCLUSIONS
In this study, we 1) Constructed brain structural and functional connectomes; 2) Explicated the brain connectome using SSAE to identify the most discriminative networks and connections supporting cognitive function to facilitate early risk stratification; and 3) Successfully predicted cognitive deficits/function, with high accuracy, sensitivity and specificity, in individual very preterm infants soon after birth using SVM. Our study shows that structural and functional brain connectome data are useful as prognostic biomarkers of neurodevelopment in preterm neonates. Additionally, we demonstrated proof of principle for applying ANN on connectome data to capture the individual variability inherent in the developing brain and improved prediction of cognitive deficit over existing conventional methods. We are conducting a larger study to validate this promising approach.Acknowledgements
No acknowledgement found.References
1. Tyson JE, Parikh NA, Langer J et al., NICHD Neonatal Research Network. Intensive care for extreme prematurity--moving beyond gestational age. N Engl J Med, 2008;358(16):1672-81.
2. Hamilton BE, Martin JA, Osterman MJ. Births: Preliminary Data for 2015. Natl Vital Stat Rep,2016; 65(3): 1-15.
3. Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology, 2014; 272(1), 29-49.
4. Kawahara J, Brown CJ, Miller SP, et al. BrainNetCNN: Convolutional neural networks for brain networks; towards predicting neurodevelopment. Neuroimage, 2016.
5. He L, Parikh NA. Aberrant Executive and Frontoparietal Functional Connectivity in Very Preterm Infants With Diffuse White Matter Abnormalities. Pediatr Neurol, 2015; 53(4), 330-337.
6. Yuan W, Treble-Barna A, Sohlberg MM, Harn B, Wade SL. Changes in Structural Connectivity Following a Cognitive Intervention in Children With Traumatic Brain Injury. Neurorehabil Neural Repair, 2017; 31(2), 190-201.
7. He H, Bai Y, Garcia EA, Li S. ADASYN: Adaptive synthetic sampling approach for imbalanced learning. Paper presented at: IEEE International Joine conference on Neural Networks. 2008; Hong Kong.
8. Hinton GE, Salakhutdinov RR. Reducing the dimensionality of data with neural networks. Science, 2006; 313(5786), 504-507.
9. Arbabshirani MR, Plis S, Sui J, Calhoun VD. Single subject prediction of brain disorders in neuroimaging: Promises and pitfalls. Neuroimage, 2017; 145(Pt B), 137-165.
10. Zhou Z, Chen Y, Ding M, Wright P, Lu Z, Liu Y. Analyzing brain networks with PCA and conditional Granger causality. Hum Brain Mapp, 2009; 30(7), 2197-2206.
11. Poline JB, Brett M. The general linear model and fMRI: does love last forever? Neuroimage, 2012; 62(2), 871-880.