5652
7T Brain MRS in HIV Infection: Effects of Cognitive Impairment1Radiology, Johns Hopkins Medical Institutions, Baltimore, MD, United States, 2Orthopedic, Johns Hopkins Medical Institutions, Baltimore, MD, United States, 3Neurology, Johns Hopkins Medical Institutions, Baltimore, MD, United States
Synopsis
Higher magnetic field such as 7T provides increased sensitivity, better signal to noise ratio and more reliable measure of the metabolite concentrations. In this study, 7T MRS was used to measure brain metabolites in HIV+ patients in 5 brain regions. Our study showed impaired neuronal integrity across the white and gray matter as well as possible impaired astrocyte osmoregulation in patients with symptomatic cognitive impairment. In conclusion, 7T MRS brain metabolites measurement can be used as reliable biomarkers for the assessment of cognitive status in HIV+ patients.
Introduction
Previous brain magnetic
resonance spectroscopy (MRS) studies at 1.5 and 3T have shown alterations in
the neuro-metabolic profile in HIV+ patients 1, 2, 3. Higher magnetic field such as 7T provides increased
sensitivity, better signal to noise ratio and more reliable measure of the metabolite
concentrations. This abstract reports on the use of 7T MRS to measure brain
metabolites in older HIV+ patients, to investigate its utility as a biomarker
for the assessment of cognition.Methods
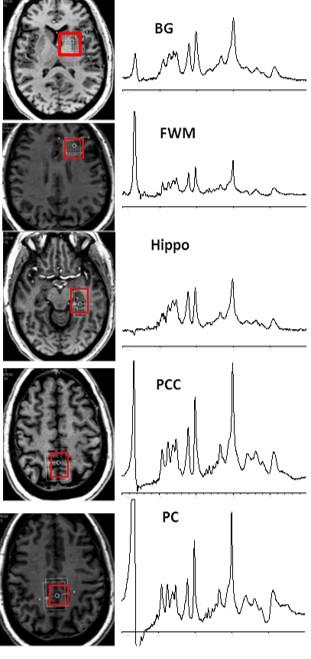
45 HIV+ patients [mean age is 58.9 years ± 5.3 SD; 33 (73%) were male] were included in this study and were stratified according to their HIV associated neurocognitive disorder (HAND) using the Frascati criteria 4. 21 HIV+ patients were classified as having symptomatic cognitive impairment [mild neurocognitive disorder (MND) and HIV associated dementia (HAD)] and 24 HIV+ were classified as asymptomatic cognitive impairment [normal cognition and asymptomatic neurocognitive impairment (ANI)]. Unlike symptomatic HAND, the cognitive impairment in ANI does not interfere with everyday functioning, although it is also characterized by cognitive impairment in at least two ability domains 4. All patients were receiving combination antiretroviral therapy (cART). Using a 7.0T Philips Achieva scanner and 32-channel head coil, brain MRI and single voxel STEAM spectra (TR/TE=3000/14 msec) were acquired from the left frontal white matter (FWM), basal ganglia (BG), precuneus (PC), posterior cingulate cortex (PCC) and hippocampus (Hippo) with and without water suppression. The voxel sizes ranged from 8 to 15 cc (Figure 1). Spectra were analyzed using LCModel 5 and quantified in institutional units (i.u., approximately mM) relative to the unsuppressed water signal. Metabolite concentrations and ratios relative to creatine (Cr) were calculated for the 2 groups. Concentration and ratio values were only included if their Cramér-Rao lower bounds (CRB) were 20% or less 5. The data was not normally distributed; therefore, comparisons of the groups’ medians and interquartile (IQR) ranges were evaluated for significant differences using a non-parametric median test.Results
There were no significant differences in demographics, CD4 count or plasma HIV RNA except for the estimated IQ which was significantly lower in the symptomatic group compared to the asymptomatic group (103.6 vs. 112.8 respectively, P=0.02). Metabolite concentrations showed no significant differences in all regions except for a trend towards significance in the median FWM total N-acetylaspartate (tNAA) which was lower in the symptomatic neurocognitive impairment group than the asymptomatic neurocognitive impairment group (7.46 vs. 7.81 mM respectively, P=0.06). Looking at the metabolites ratios, on comparing the patients with symptomatic vs. without symptomatic impairment, the frontal white matter, showed significant differences in the median NAA/Cr (1.21 vs. 1.30 respectively, P=0.005), the median tNAA/Cr (1.46 vs. 1.56 respectively, p=.005) and the median myoinositol/Cr (mI/Cr) (0.97 vs. 1.03 respectively, P=0.02). In the posterior cingulate, there was a significant difference in median NAA/Cr between the symptomatic vs. asymptomatic cognitive impairment (1.16 vs. 1.21 respectively, P=0.01) and median tNAA/Cr (1.32 vs. 1.40 respectively, P=0.02), and median mI/Cr (0.78 vs. 0.87 respectively, P=0.002). In the precuneus, there were significant differences in median NAA/Cr between the symptomatic vs. asymptomatic cognitive impairment (1.14 vs. 1.23 respectively, P=0.02) and median glutamate/Cr (Glu/Cr) (1.12 vs. 1.24 respectively, P=0.01). There was no significant differences in basal ganglia or hippocampus metabolite ratios.Discussion
In agreement to previous studies, our study showed impaired neuronal integrity across the white and gray matter regions as reflected by the decreased NAA/Cr and Glu/Cr as well as possible impaired astrocyte osmoregulation as reflected by decreased mI/Cr in HIV patients with symptomatic cognitive impairment.Conclusion
7T MRS brain metabolites measurement can be used as reliable biomarkers for the assessment of cognitive status in HIV+ patients.Acknowledgements
This work was supported in part by RO1NS081196References
1. Mohamed MA, Barker PB, Skolasky RL. Brain metabolism and cognitive impairment in HIV infection: a 3-T magnetic resonance spectroscopy study et al. Magn Reson Imaging 2010; 28 (9):1251-7.
2. Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients. NMR Biomed 2009; 22 (3):326-31.
3. Bairwa D, Kumar V, Vyas S, et al. Case control study: magnetic resonance spectroscopy of brain in HIV infected patients. BMC Neurol. 2016; 16:99.
4. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007; 69(18):1789:99
5. Provencher SW, Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30(6):672:9.