5651
NEURO 2D CORRELATED SPECTROSCOPY IDENTIFIES NEURO DEREGULATION IN SOLDIERS EXPOSED TO BLAST PRIOR TO DISCERNIBLE CHANGES BY CONVENTIONAL IMAGING1Translational Research Institute, Woolloongabba, Australia, 2Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 3University of Newcastle, Australia, 4Institute for Health and Biomedical Innovation, Brisbane, Australia, 5Department of Defence, Australian Government, Sydney, Australia
Synopsis
This pilot study reports clear deregulation in the neurochemistry of defense personnel exposed to repeated blast. The changes recorded are different to those reported for mTBI, PTSD and chronic pain. No differences between blast exposed and healthy were recorded by MRI sequences T1WI, FLAIR or SWI. In vivo neuro 2D spectroscopy recorded deregulation with PC and GPC, NAA and GABA all decreased compared to the healthy non exposed brain. We we did not observe any changes in the fucosolated glycans, which are reflective of pain, repetitive brain injury and/or cognitive deficit.
Introduction
Blast injury, depending on length and level of exposure, can result in the full spectrum of traumatic brain injury (TBI) from mild to severe TBI and is common in combat. It is estimated that 60-80% of the soldiers, exposed to a blast, will acquire a TBI [1] and that injury can occur from a blast, without physical injury [2]. The sequelae of blast induced mTBI is similar to non-blast related mTBI with the majority of individuals suffering no long term effects. There is a risk of post concussive syndrome, chronic traumatic encephalopathy (CTE) (repeated blast), and/or PTSD. In a retrospective review of the medical records of 27,169 U.S. Army Special Operations Command personnel, respondents with blast associated mTBI (OR = 4.23) were at significantly greater risk of reporting PTSD symptoms [3]. It is hypothesised that 2D Correlated SpectroscopY (COSY) will identify early neurochemical deregulation in the brain of those exposed to blast prior to changes recorded by clinical MRI sequences.Purpose
Determine, using neuro 1D and 2D COSY, if there is neuro deregulation in subjects exposed to chronic low dose blast injury as a result of using repeated artillery firing or explosive methods of entry (breaching) or common sources of blast exposure within the defense forces. The results will be compared with standard clinical MRI sequences, T1WI, FLAIR and SWI.Methods
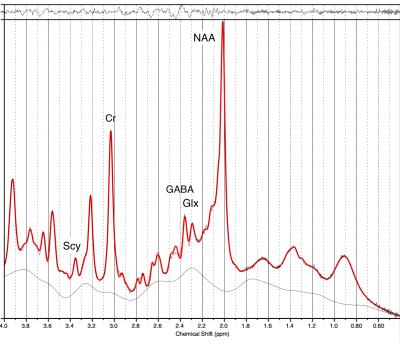
Age matched male participants, healthy (n=8) and blast exposed (n=8), were screened to assess pre-existing exposure to traumatic brain injury using the Ohio State University Traumatic Brain Injury Identification Method [4]. The Life Events Checklist for DSM-5 [5] was administered to ascertain lifetime exposure to traumatic events known potentially to cause post-traumatic stress disorder. Participants were then assessed using the Structured Clinical Interview for DSM-5, [6] and excluded if they met diagnostic criteria for any mental health problems, including PTSD. One-dimensional (1D) and two-dimensional (2D) localised COrrelation SpectroscopY (L-COSY) MRS was acquired from the posterior cingulate gyrus (PCG) using a 3T Prisma MRI scanner (Siemens Healthcare, Erlangen, Germany). MRS was performed on 8 blast exposed participants. Comparisons were made with a healthy, approximately age and gender-matched control group (n=8). Data were acquired from a 3x3x3 cm3 voxel. 1D profiles were analysed with LCModel (v6.2-2B) using water normalization. Felix 2007 software was used for the processing and analysis of the 2D L-COSY data. The creatine methyl resonance (3.02-3.02ppm) was used as the internal chemical shift reference and for volume normalisation of 2D L-COSY. Average peak areas/volumes were calculated for each assigned metabolite and compared using a t-test. MRI sequences 3D MP-RAGE (TR/TE/TI = 2530/3.5/1100msec), FLAIR (TR/TE/TI =5000/394/1800ms, and Siemens 3D SWI(TR/TE/FA = 20/27/15msec) were recorded using 64 channel headcoil and visually inspected for lesions.Results
None of the blast exposed patients showed structural brain abnormalities on T1 images, nor abnormalities with SWI or FLAIR. However there were significant reductions in neurochemicals recorded in the 1D and 2D spectra of those exposed to blast. The sum of phosphoryl choline (PC) and Glycero-phosphocholine (GPC) was consistently reduced in the blast exposed cohort compared to controls with a reduction of 19% (p<0.05) (1D data) and 18% (p<0.005) (2D data). LC model showed reduction in NAA at 2.02ppm of 17% (p<0.01) in 1D data but this was not significant in the 2D data sets (diagonal peak at 2.02ppm). The NAA cross peak at (F2: 4.36, F1:2.55ppm) was however decreased by 8% (p<0.03) in the blast cohort . The 1D data showed a decrease of 26% (p<0.01) in phosphocreatine (PCr), but as the 2D spectra were normalised to Creatine, comparison with 2D COSY not possible. 1D spectra showed a 23% decrease (p<0.01) in GABA, which was not reflected in the 2D spectra. There were significant a decreases in two, unassigned peaks in the 2D-COSY spectra: (2.36,2.67ppm) by 11.6% (p<0.05) and (3.18,3.54ppm) by 15% (<0.05).
Discussion and Conclusion
Neuro 1D and 2D in vivo spectroscopy show quite large and statistically significant changes in blast exposed subjects not recorded by imaging techniques. Compared to normal healthy controls, PC and GPC were reduced reflecting reduced membrane synthesis; decreases in NAA and GABA suggesting neuronal activity is affected and the decrease in PCr suggests mitochondrial dysfunction. No changes were recorded in the fucosolated glycans[7], which are reflective of pain [8], CTE [9] and cognitive deficit.[10]. This pilot study reports clear deregulation in the neurochemistry of defense personal exposed to repeated blast. The changes are different to those reported for mTBI, PTSD and chronic pain.Acknowledgements
Funding was provided by US and Australian DoD CDMRP (W81XWH-10-1-0835), The authors acknowledge the support of team members Valerie Graves and Lisa Rich for project management, Dr Saad Ramadan, for development of the L-COSY sequence, and radiographers Jameen Arm and Kylie Waters for implementing the protocols and running the scanner. We thank Defence personnel Drs Helen Cartledge, Patrick Cullinan and Amanda Toman for operational aspects.References
1. Wilk, J.E., et al.,
Mild traumatic brain injury (concussion)
during combat: lack of association of blast mechanism with persistent
postconcussive symptoms. J Head Trauma Rehabil, 2010. 25(1): p. 9-14.
2. Bhattacharjee, Y., Neuroscience. Shell shock revisited: solving
the puzzle of blast trauma. Science, 2008. 319(5862): p. 406-8.
3. Kontos, A.P., et
al., Residual effects of combat-related
mild traumatic brain injury. J Neurotrauma, 2013. 30(8): p. 680-6.
4. Corrigan, J.D.,
Bogner, J. A. , Initial reliability and
validity of the OSU TBI Identification Method. Journal of Head Trauma
Rehabilitation, 2007. 22(6): p.
318-329.
5. Weathers, M.B.,
Blake, D.D., Schnurr, P.P., Kaloupek, D.G., Marx, B.P. and Keane, T.M. The Life Events Checklist for DSM-5 (LEC-5).
Instrument available from the National Center for PTSD at www.ptsd.va.gov 2013.
6. First, M.B., Williams,
J. B. W., Karg, R. S., Spitzer, R. L. , Structured
Clinical Interview for DSM-5 – Research Version (SCID-5 for DSM-5, Research
Version; SCID-5 RV) 2015, Arlington, VA American Psychiatric Association.
7. Mountford, C., et
al., Six fucose-alpha(1-2) sugars and
alpha-fucose assigned in the human brain using in vivo two-dimensional MRS.
NMR Biomed, 2015. 28(3): p. 291-6.
8. Quadrelli SG, R.S.,
Lin A, Dimitrikof JD, Mountford CE,, α-Fucose increased in the brain of Chronic Pelvic Pain
Syndrome patients with inflammation at onset recorded by 2D L-COSY, in ISMRM. 2014: Milan.
9. Lin, A.P., et al., Changes in the neurochemistry of athletes
with repetitive brain trauma: preliminary results using localized correlated
spectroscopy. Alzheimer's Research & Therapy, 2015. 7(1): p. 13.
10. Murrey, H.E., et
al., Identification of the
Plasticity-Relevant Fucose-α(1-2)-Galactose Proteome from Mouse Olfactory Bulb.
Biochemistry, 2009. 48: p.
7261–7270.