5645
The subjective intensity of pain in healthy subjects is inversely correlate with posterior insular GABA levels1Neuroimaging, Central Institute of Mental Health, Mannheim, Germany, 2Center for Biomedicine and Medical Technology Mannheim, Medical Faculty Mannheim, Mannheim, Germany, 3Psychosomatic Medicine and Psychotherapy, Central Institute of Mental Health, Mannheim, Germany
Synopsis
We present single voxel MEGA-PRESS MRS data from the posterior insula of 20 healthy women demonstrating a significant association of GABA and the subjective pain thresholds. These findings are in good agreement with the postulated role of the posterior insula for pain information processing. In this region pain is first processed and the sensory aspects of pain perception is elaborated and then conveyed to the anterior insula where it is related to emotional and cognitive aspects of pain perception. The data corroborate that GABA levels seem to be an important mediator for pain perception.
Introduction:
The insula is known as a
multisensory organ and is a key region in pain processing, as it is involved in
both sensory-discriminative and affective-motivational aspects of pain
processing 1, 2. According to recent findings in spatiotemporal pain
processing, pain information is first processed in the posterior insula, where
the sensory aspects of pain perception is elaborated and then conveyed to the
anterior insula where it is related to emotional and cognitive aspects of pain
perception3,4. The insula is the region where the strongest pain
related activation is observed1 and takes part in the encoding of
pain intensity5,6.
Studies in animals underline the
role of glutamate (Glu) and GABA levels in setting the pain threshold in the
insular cortex7.
Although MRS studies pointed out elevated
ratio of excitatory to inhibitory neurotransmitters in patients with chronic
pain [9], there are only few studies reporting insular neurotransmitters in
healthy controls (HC). Zunhammer et al. found that pooled Glx-levels across
pain related areas were positively associated with pain sensitivity8.
We hypothesized that GABA and Glu levels in the
posterior insula correlate with the individual pain sensitivity in healthy
subjects. We hypothesized that inter-individual pain sensitivity could be
reflected in the posterior insula. The study aimed to investigate the role of
the posterior insular GABA and Glu levels in pain processing in healthy female subjects.Methods
Twenty healthy female individuals (mean age 24.4 ± 4.8 years) underwent single voxel magnetic resonance spectroscopy at 3Tesla. In vivo single voxel MR spectra were acquired using a MEGA-PRESS editing sequence with the following parameters: echo time TE=68ms, repetition time TR=3s. The editing pulse (Gauss shape, 20.36 ms length, bandwidth (FWHM): 44 Hz) was switched between 1.9 and 1.5 ppm (2nd editing frequency 1.5 ppm) alternating every excitation. This editing scheme diminishes contamination by nearby macromolecule resonances10. In order to achieve a sufficient signal-to-noise ratio, we chose a bigger voxel size than just the posterior insula’s size but did not include the anterior insula in the voxel (figure 1a). Mechanical pain sensitivity was experimentally assessed with pinprick-stimuli on a numeric rating scale.Results
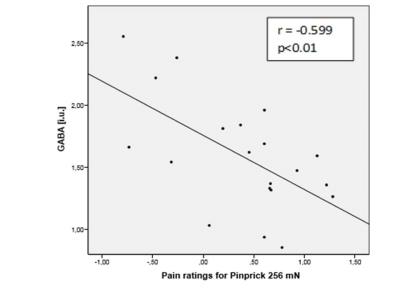
Clinical pain measures and metabolite levels were correlated using a two-tailed Pearson correlation, with significance level set to p<0.05. IBM SPSS (Statistical Package oft the Social Sciences) Statistics 23 was used for the statistical analysis. Ratings of perceived intensity of pinprick of 256mN and 512mN stimuli inversely correlated with GABA levels in the posterior insula: 256mN (r = -0.599; p<0.01), and 512mN (r = -0.542; p<0.05) (figure 1b). These pinprick pain ratings also positively correlated with the ratio Glu/GABA. There was no correlation for glutamate levels and no significant correlation for pinprick of lower forces than 256m.Discussion
Our finding
of an inverse correlation of PI GABA with pain sensitivity is concordant with
previous findings in diabetic neuropathy showing both elevated Glx and reduced
GABA levels in the posterior insula9,11. Furthermore, the results are consistent with
studies in rats, showing that locally increasing GABA levels in the rostral
agranular cortex produced analgesia in rats12. These results were
confirmed by a recent finding that in naive rats, raising and decreasing GABA
levels in the rostral insular cortex directly decreased or increased their pain
sensitivity, respectively, and inversely for Glu13. Conclusion
The results of our study support the hypothesis that inhibitory neurotransmitter levels and/or the ratio of excitatory to inhibitory levels in the posterior insula are related to individual differences in pain sensitivity. These results are in line with chronic pain studies, where patients with chronic pain syndromes had elevated excitatory/inhibitory metabolite ratios in the insular cortex.Acknowledgements
This work was supported by the German Research Foundation, KFO256, Project IP6References
1. Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. European journal of pain 2005;9(4):463-484.
2. Cerliani L, Thomas RM, Jbabdi S, Siero JC, Nanetti L, Crippa A, Gazzola V, D'Arceuil H, Keysers C. Probabilistic tractography recovers a rostrocaudal trajectory of connectivity variability in the human insular cortex. Human brain mapping 2012;33(9):2005-2034.
3. Bastuji H, Frot M, Perchet C, Magnin M, Garcia-Larrea L. Pain networks from the inside: Spatiotemporal analysis of brain responses leading from nociception to conscious perception. Human brain mapping 2016.
4. Frot M, Faillenot I, Mauguiere F. Processing of nociceptive input from posterior to anterior insula in humans. Human brain mapping 2014.
5. Frot M, Magnin M, Mauguiere F, Garcia-Larrea L. Human SII and posterior insula differently encode thermal laser stimuli. Cerebral cortex 2007;17(3):610-620.
6. Iannetti GD, Zambreanu L, Cruccu G, Tracey I. Operculoinsular cortex encodes pain intensity at the earliest stages of cortical processing as indicated by amplitude of laser-evoked potentials in humans. Neuroscience 2005;131(1):199-208.
7. Watson CJ. Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain 2016.
8. Zunhammer M, Schweizer LM, Witte V, Harris RE, Bingel U, Schmidt-Wilcke T. Combined glutamate and glutamine levels in pain-processing brain regions are associated with individual pain sensitivity. Pain 2016;157(10):2248-2256.
9. Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 2013;8(3):576-593.
10. Henry PG, Dautry C, Hantraye P, Bloch G. Brain GABA editing without macromolecule contamination. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine 2001;45(3):517-520.
11. Harris RE, Clauw DJ. Imaging central neurochemical alterations in chronic pain with proton magnetic resonance spectroscopy. Neuroscience letters 2012;520(2):192-196.
12. Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature 2003;424(6946):316-320.
13. Watson CJ. Insular balance of glutamatergic and GABAergic signaling modulates pain processing. Pain 2016.