5644
Improving the detection of scalar-coupled resonances at short and moderate echo times for in vivo rat MRS at 9.4 T1Department of Electronic Sciense, Xiamen University, Xiamen, People's Republic of China, 2Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University, Baltimore, MD, United States, 3F. M. Kirby Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 4High Magnetic Field Laboratory, CAS Center for Excellence in Brain Science, Chinese Academy of Sciences, Hefei, People's Republic of China
Synopsis
Conventional localized 1H MRS pulse sequences, such as PRESS and STEAM, generally suffer from J coupling modulations which can aggravate attenuation of multiplet resonances during echo times. Here, the “perfect echo” module combined with an optimized localization scheme is utilized for in-phase single-voxel in vivo MRS at 9.4 T. The relative signal intensities of multiplet to singlet resonances acquired at short and moderate echo times increase substantially in comparison with those at PRESS spectra. Therefore, direct MRS quantification of many important metabolites, such as glutamine, glutamate, γ-aminobutyrate, aspartate, and myo-inositol, may be improved.
Purpose
MR spectroscopy, as a noninvasive technique for in vivo metabolic and biochemical detections, has now been widely utilized for preclinical and clinical studies. Currently, Point RESolved Spectroscopy sequence (PRESS) and Stimulated Echo Acquisition Mode (STEAM) are the two commonly used single voxel 1H MRS pulse sequences. However, they both suffer from scalar coupling (J) modulations during echo times, thus hampering direct quantification of multiplet resonances. In the Carr-Purcell-Meiboom-Gill (CPMG) experiments1, J modulations within echo time can be suppressed through repeating 180° pulses with very short interpulse intervals (τ ≪ 1/∆ϑ, ∆ϑ is the chemical-shift difference between coupled resonances). Additionally, the “perfect echo”2, achieved by placing a 90° pulse in the midpoint of two spin echoes, can also refocus evolution of scalar coupling with longer interpulse intervals (τ ≪ 1/J). Therefore, the radio frequency (RF) power deposition can be greatly reduced by the “perfect echo” in comparison with CPMG under identical echo times. The “perfect echo” has been previously utilized to the acquisition of magnetic resonance spectroscopic imaging3. In this study, the “perfect echo” module combined with an optimized localization scheme is introduced to achieve In-Phase Point RESolved Spectroscopy (IP-PRESS) for in vivo detection.Methods
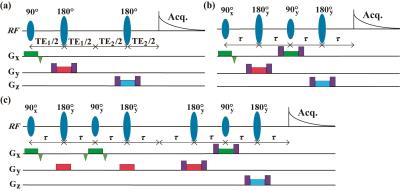
The proposed pulse sequences (IP-PRESS), modified from conventional PRESS sequence, are shown in Fig. 1. Commonly, J values within metabolites are smaller than 15 Hz. As a result, the achievable maximum TE in the single echo IP-PRESS (Fig. 1(b)) can be about 30 ms. For longer echo times, the “perfect echo” need to be repeated for satisfactory suppression of J modulation, and the double echo IP-PRESS is presented in Fig. 1(c) for example. A healthy rat was scanned to demonstrate the in vivo performance of IP-PRESS. The voxel size was 5.0×5.0×7.0 mm3. Average number were 128 for PRESS and 256 for IP-PRESS with TR = 2.5 s. For PRESS, the minimum TE (13 ms) was achieved by using TE1 = 7 ms and TE2 = 6 ms. Other TEs were achieved by setting TE1 = 10 ms, with TE2 values of 10, 20, 30, and 50 ms, respectively. For IP-PRESS, short TEs (14, 20, and 30 ms) were achieved by single echo IP-PRESS, and moderate TEs (40, and 60 ms) were achieved by double echo IP-PRESS. Experiments were performed on 9.4 T/400 mm animal scanners (Agilent Technologies, Inc., Santa Clara, CA, USA), using volume RF coils. The variable power and optimized relaxation delays (VAPOR) module provided by vendor was used to suppress the strong water signal.Results and discussion
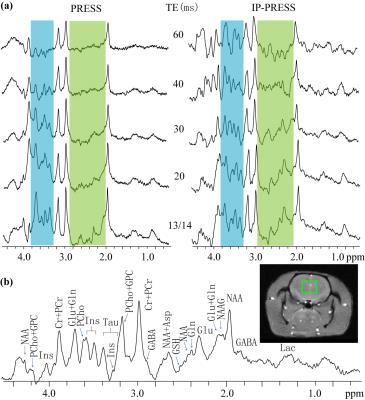
Figure 2 presents PRESS and IP-PRESS rat brain spectra acquired at 9.4 T. The blue and green shaded regions in Fig. 2(a) mainly comprise of multiplet resonances. Due to the evolution of scalar coupling, intensities of these multiplet resonances in PRESS spectra suffer from significant reduction even at the shortest echo time (13 ms), and decay nearly to zero at 60 ms. By refocusing the scalar coupling evolution, relative signal intensities of multiplet resonances to those of singlet resonances are greatly improved in IP-PRESS spectra and the multiplet signals can still be recognizable at 60 ms. Therefore, recognition and analysis of multiplet signals can be more feasible in IP-PRESS spectra. The signal intensity and baseline performance of IP-PRESS spectrum at 30 ms are favorable, and the metabolite assignment on this spectrum is shown in Fig. 2(b). A limitation of IP-PRESS pulse sequences lies in that only half of the magnetization from designated voxel can be retained. Additionally, the RF power deposition will also increase in either single or multi echo IP-PRESS in comparison with PRESS.Conclusion
We have developed a new pulse sequence for in-phase single-voxel MRS. In vivo rate brain spectra acquired at short and moderate echo times at 9.4 T demonstrate that relative intensities of scalar coupling resonances to singlet resonances are significantly enhanced. Therefore, the proposed pulse sequence may provide an approach for better quantification and fitting of scalar coupled peaks for proton MRS in brain.Acknowledgements
This work was partially supported by the National Natural Science Foundation of China under Grants 11105114 and 11375147, and the Natural Science Foundation of Fujian Province of China under Grant 2014J05012, and the State Scholarship Fund of China (No. 201606310173).References
1. Hennig J, Thiel T, Speck O. Improved sensitivity to overlapping multiplet signals in in vivo proton spectroscopy using a multiecho volume selective (CPRESS) experiment. Magn Reson Med 1997;37(6):816-820.
2. Lin YQ, Lin LJ, Wei ZL, Zhong JH, Chen Z. Localized one-dimensional single voxel magnetic resonance spectroscopy without J coupling modulations. Magn Reson Med DOI 10.1002/mrm.26066.
3. Pan J.W., Avdievich N., Hetherington H.P. J-refocused coherence transfer spectroscopic imaging at 7 T in human brain. Magn Reson Med 2010; 64(5):1237–1246.
Figures