5641
Cerebral Metabolite Changes and Cognitive Clinical Correlates in Perinatally HIV-infected Young Adults1Radiological Sciences, UCLA School of Medicine, Los Angeles, CA, United States, 2Pediatrics, Harbor-UCLA Medical Center, Torrance, CA, United States, 3Semel Institute for Neuroscience and Human Behavior, UCLA School of Medicine, Los Angeles, CA, United States, 4Infectious disease-Pediatrics, Miller Children's Hospital, Long Beach, CA, United States, 5Radiology, Harbor-UCLA Medical Center, Torrance, CA, United States, 6Pediatrics, UCLA School of Medicine, Los Angeles, CA, United States, 7Pediatrics, Keck School of Medicine of USC, Los Angeles, CA, United States, 8Psychiatry and Biobehavioral Sciences, UCLA School of Medicine, Los Angeles, CA, United States
Synopsis
A recently implemented 5D echo-planar J-resolved spectroscopic sequence using 8x acceleration and compressed sensing reconstruction was evaluated in 7 perinatally infected and 8 healthy youths. Selected metabolite ratios with respect to Cr were detected bilaterally in the basal ganglia, anterior insular cortex, posterior insular cortex, frontal white and occipital/frontal gray regions of the two groups. Statistically significant differences were found between metabolite ratios (/Cr) of HIV-infected youing adults and healthy control subjects in the occipital gray N-acetylaspartate, right basal ganglia glutamine/glutamate, left anterior insular cortex choline, and left posterior insular cortex. Also, our pilot findings suggest a possible difference in energy metabolism between perinatally HIV-infected young adults and controls without HIV. The metabolite ratios correlated with neuropsychological test scores showing cognitive impairment as result of HIV-infection and/or long term ART.
Introduction
Perinatally HIV-infected young adults represent a highly unique population, having been infected at a time of immune compromise (in utero and at birth) and having experienced many years of antiretroviral therapy (ART)1. Despite the success of ART2, signs of neurocognitive compromise are common. Hence, non-invasive monitoring is needed to ensure early detection of neurocognitive disorders or other CNS abnormalities arising from HIV infection and its treatment. MRS can detect metabolic changes in cerebral metabolite resulting from HIV infections3,4. One dimensional (1D) MRS has previously been used to investigate the differences between healthy children and pediatric patients with perinatal HIV5-10. The purpose of this study was to evaluate changes between healthy and perinatally HIV-infected young adults using a semi-laser based accelerated five-dimensional (5D) echo-planar J-resolved spectroscopic imaging (EP-JRESI)11 sequence. We also explored the relationship between brain metabolite ratios and cognitive performance as well as the clinical variables.Materials and Methods:
We investigated seven perinatally HIV infected patients (age 21.1± 2.0years) and eight healthy controls (HC) (age 20.8± 1.8years). All data were collected on a Siemens 3T Prisma MRI scanner using a 16 channel head receive coil. The following parameters were used for water-suppressed 5D EP-JRESI: TR/TE=1.2s/40ms, voxel resolution=1.13x1.13x1.5cm3, 64 ∆t1 increments, 512 bipolar echo pair, FOV=18x18x12cm3, 1 average, non-uniform sampling (NUS)= 12.5% with a scan time≈20min. A maximum echo sampling scheme was applied and after post-processing12 bandwidth was ±250Hz along F1 and 1190Hz along F2. This was followed by a non water-suppressed scan with only the first t1. The 5D EP-JRESI data were reconstructed as discussed previously11. The undersampled data were reconstructed using a modified Split Bregman algorithm13 which solves the optimization problem, $$\min_{u} TV(u) \quad \text{s.t.} \|R\mathcal{F}u - f\|_2^2 < \sigma^2$$ where u is the reconstructed data, f is the undersampled data, $$$\sigma^2$$$ is an estimate of the noise variance, TV is the total variation norm, R is the NUS masking operator, and $$$\mathcal{F}$$$ is the Fourier transform operator. Acquired data were post-processed with a custom MATLAB-based program and metabolite ratios with respect to the 3.0 ppm creatine (Cr) peak were calculated using peak integration. The metabolite differences between the two groups were tested with a two-tailed t-test. To explore for any relationship between the metabolite ratios and clinical parameters along with the Neuropsychological (NP) Raw Test Scores data from a battery of tests including verbal fluency (FLUENCY), trail making test (TRAILS), stroop word score (STROOP WORDS), and WMS-III spatial span (WMS) a Pearson correlation was performed on the patient data from the regions where we found metabolite differences. All statistical analysis was done using the SPSS software.Results:
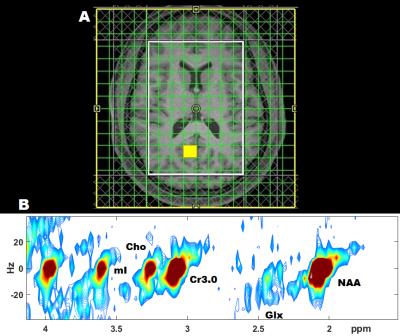
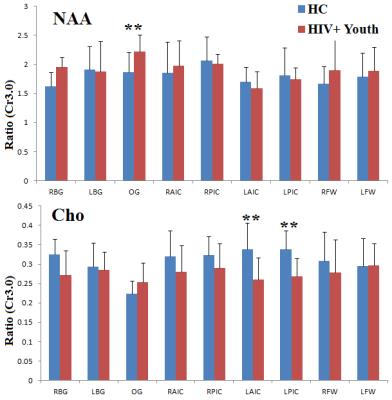
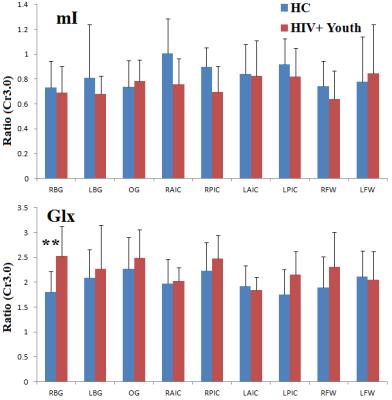
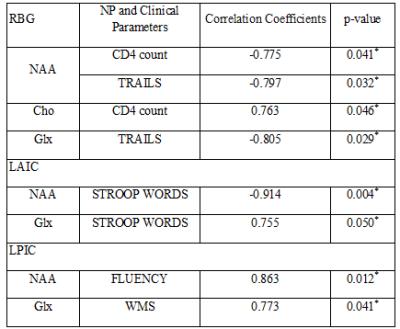
Fig. 1(A) shows the PRESS-localized volume of interest (VOI) on a T1-weighted axial brain MRI of a 20-year-old HIV-infected youth. Representative 2D J-resolved spectra extracted from the right occipital gray (ROG) regions of the same subject is shown in Figure 1(B). Fig. 2 and Fig. 3 show selected metabolite ratios with respect to Cr for the two groups. Statistically significant differences were found between HIV-infected young adults and HC subjects in the occipital gray (OG) N-acetylaspartate(NAA)/Cr (1.86[0.35] vs 2.23[0.28]), right basal ganglia (RBG) Glx/Cr (1.80[0.42] vs 2.53[0.59]), left anterior insular cortex (LAIC) choline(Cho)/Cr (0.34[0.07] vs 0.26[0.06]), and left posterior insular cortex (LPIC) Cho/Cr (0.34[0.05] vs 0.27[0.05]) ratios. Table 2 shows the results from the correlation analysis between the metabolite ratios versus the clinical and NP tests.Discussion:
Our pilot data showed increased Glx/Cr, NAA/Cr and decreased Cho/Cr in agreement with some of the previous studies in perinatally HIV-infected children14-16. Assuming the mechanisms for glutamate (Glu) transportation and uptake are unhindered, an increase in Glu can cause increases in glutamine (Gln) and increased asparate which indirectly may cause NAA to increase14. Both Glx and NAA have been reported to decrease in in different regions of the brain in adults and in children15-18 when neurological disorders were present. Our findings suggest a possible difference in energy metabolism between perinatally HIV-infected young adults despite many years of therapy. Also, to be noted that 3 of the 7 HIV-infected youth had a diagnosis of HIV encephalopathy. The negative and positive correlations of CD4 count with NAA and Cho respectively in HIV youth are reflective of our finding in metabolite changes. The metabolite correlation with NP scores showed cognitive impairment as result of HIV-infection and/or long term ART.Conclusion:
Our findings are indicative of an abnormal energy metabolism in the brain of the perinatally HIV-infected young adults despite long term A=RT. Future studies using a larger sample size are required to further authenticate the observations of the current study.Acknowledgements
This research was supported by two NIH grants: 1R21-NS090956 (PI: Sarma) and 5R21NS080649-02 (PI: Thomas). Authors thank Z. Iqbal for his help.References
1. Patel K, Ming X, Williams PL, et al. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23(14):1893-901.
2. Tardieu M, Mayaux MJ, Seibel N, et al. Cognitive assessment of school-age children infected with maternally transmitted human immunodeficiency virus type 1. J Pediatr. 1995;126(3):375-9.
3. Catani M, Cherubini A, Howard R, Tarducci R, Pelliccioli GP, Piccirilli M, Gobbi G, Senin U, Mecocci P. (1)H-MR spectroscopy differentiates mild cognitive impairment from normal brain aging. Neuroreport 2001; 12:2315–2317.
4. Laubenberger J, Haussinger D, Bayer S, et al. HIV-related metabolic abnormalities in the brain: Depiction with proton MR spectroscopy with short echo times. Radiology. 1996;199:805–810.
5. Salvan AM, Lamoureux S, Michel G, et al. Localized proton magnetic resonance spectroscopy of the brain in children infected with human immunodeficiency virus with and without encephalopathy. Pediatric research. 1998;44:755–762.
6. Pavlakis SG, Lu D, Frank Y, et al. Magnetic resonance spectroscopy in childhood AIDS encephalopathy. Pediatric neurology. 1995;12:277–282.
7. Pavlakis SG, Lu D, Frank Y, et al. Brain lactate and N-acetylaspartate in pediatric AIDS encephalopathy. American journal of neuroradiology. 1998;19:383–385.
8. Keller M, Venkatraman T, Thomas A, et al. Altered neurometabolite development in HIV-infected children Correlation with neuropsychological tests. Neurology. 2004;62:1810–1817.
9. Keller M, Venkatraman T, Thomas M, et al. Cerebral metabolites in HIV-infected children followed for 10 months with 1H-MRS. Neurology. 2006; 66:874–879.
10. Lu D, Pavlakis SG, Frank Y, et al. Proton MR spectroscopy of the basal ganglia in healthy children and children with AIDS. Radiology. 1996; 199:423–428.
11. Wilson NE, Iqbal Z, Burns BL, et al. Accelerated five-dimensional echo planar Jresolved spectroscopic imaging: Implementation and pilot validation in human brain. Magnetic Resonance in Medicine. 2016;75:42–51.
12. Schulte RF, Lange T, Beck J, et al. Improved twodimensional J-resolved spectroscopy. NMR Biomed. 2006;19:264–270.
13. Goldstein T, Osher S. The split Bregman method for L1 regularized problems. SIAM J Imaging Sci. 2009;2:323–343.
14. Iqbal Z, Wilson NE, Keller MA, et al. Pilot Assessment of Brain Metabolism in Perinatally HIV-Infected Youths Using Accelerated 5D Echo Planar J-Resolved Spectroscopic Imaging. PLoS One. 2016;13:11(9):e0162810.
15. Nagarajan R, Sarma MK, Thomas MA, et al. uropsychological function and cerebral metabolites in HIV-infected youth. J Neuroimmune Pharmacol. 2012;7(4):981-990.
16. Keller MA, Venkatraman TN, Thomas A, et al. Altered neurometabolite development in HIV-infected children: correlation with neuropsychological tests. Neurology. 2004;62(10):1810-1817.
17. Sailasuta N, Shriner K, Ross B. Evidence of reduced glutamate in the frontal lobe of HIV-seropositive patients.NMR in biomedicine 2009; 22:326–331.
18. Ernst T, Jiang CS, Nakama H, et al. Lower brain glutamate is associated with cognitive deficits in HIV patients: A new mechanism for HIV-associated neurocognitive disorder. Journal of Magnetic Resonance Imaging 2010; 32:1045–1053.
Figures