5636
Reliability of in vivo Glx measurements from GABA-edited MRS at 3T1Radiology, University of Calgary, Calgary, AB, Canada, 2CAIR Program, Alberta Children's Hospital Research Institute and Hotchkiss Brain Institute, University of Calgary, 3Department of Neuroimaging, Institute of Psychiatry, Psychology & Neuroscience, King's College London, 4Radiology, The Johns Hopkins School of Medicine, 5F. M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, 6General Electric Healthcare, 7School of Psychology, University of Birmingham
Synopsis
Mixed glutamate/glutamine (GLX) signal contributes to spectra acquired for GABA editing, both as a co-edited peak in the difference spectrum and in the OFF subspectrum. GLX results are often included in GABA studies, but the reliability of these metrics has received little attention. In this study, we examine the relationship between GLX measures, using a short-TE PRESS as a “gold standard”, and comparing GLX measured from the co-edited peak and the OFF subspectrum from typical GABA+ and macromolecule-suppressed GABA acquisitions.
Purpose
Glutamate (Glu) is the primary excitatory neurotransmitter and GABA is the primary inhibitory neurotransmitter in the human brain. While the individual metabolite levels can be informative, measuring both GABA and glutamate can provide insight to the inhibitory-excitatory balance. Both glutamate and GABA are present on the 1H spectrum. Due to the spectral overlap of glutamate and glutamine, GLX, representing the combination of glutamate+glutamine is typically reported. GLX is typically measured using a conventional PRESS sequence. Due to limited chemical shift displacement and overlap with more abundant metabolites, editing is required in order to measure GABA; the most typical method is using a MEGA-PRESS acquisition [1]. The two typical GABA-edited implementations are (1) the traditional MEGA-PRESS acquisition, spectra from which contain macromolecule (MM) contamination due to the limited selectivity of the editing pulses (GABA+) or (2) the MM-suppressed implementation, which applies the “OFF” editing pulse symmetric about the MM peak to limit MM contamination [2,3].
Because the balance of inhibition and excitation is of great interest in many studies, it is desirable to acquire this data simultaneously, not only to reduce scan duration but also to ensure measurements are made in the same tissue. Within the GABA-edited data, there is information about GLX, both as a co-edited peak in the difference spectrum and within the OFF-subspectrum. The purpose of this study was to examine the reliability of these measures of GLX (OFF-subspecturm GLX and co-edited GLX peak) from GABA-edited data (both GABA+ and MM-suppressed GABA methods), using a conventional PRESS acquisition as a “gold standard” for the GLX measures.
Methods
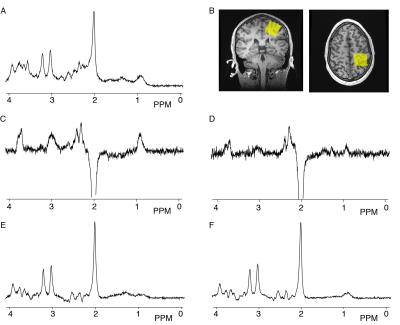
Ten right-handed males (average age 22.6±4.06 y) with no history of neurological, psychological, or psychiatric illnesses were scanned on a 3T GE 750w scanner (General Electric Healthcare, USA) with a 32-channel head coil twice within a week at a similar time during the day and using the same protocol. Data were acquired from a 3x3x3cm3 voxel in the left sensorimotor cortex. Three MRS acquisitions were performed: (1) short-echo PRESS (SE-PRESS; TR/TE = 1.8s/35 ms, 64 averages. (2) GABA+ (TR/TE = 1.8s/68ms, 320 averages, editing pulses of 14 ms at 1.9 ppm (‘ON’) and 7.46 ppm (‘OFF’). (3) MM-suppressed GABA (TR/TE = 1.8s/80ms, 20 ms editing pulses; ON at 1.9 ppm and OFF at 1.5 ppm). Data were analyzed using Tarquin(4.3.10) [4], using standard settings, including retrospective frequency correction. GLX levels were quantified using the unsuppressed water signal measured from the same volume as a reference. The co-edited GLX signal and the GLX signal from the OFF subspectrum from both the GABA+ and the MM-suppressed GABA data were compared to the GLX from the SE-PRESS data using correlation analysis. Statistics were performed using SPSS version 24.Results
One SE-PRESS dataset was not available due to technical difficulty and one was discarded due to poor spectral quality. Example spectra from one session of one participant are shown in Figure 1. Among the 18 complete datasets, significant correlations were seen between the GLX measures from the SE-PRESS and co-edited GABA+ (r=0.59, p=0.010) and between SE-PRESS and co-edited MM-suppressed GABA r=0.55 (p=0.019). However, the correlation between the SE-PRESS and OFF sub-spectrum for the GABA+ was non-significant (r=0.36, p=0.16), while the correlation between the SE-PRESS and OFF sub-spectrum MM-suppressed was significant (r=0.645, p=0.004).Discussion
As it is often desirable to simultaneously quantify GLX and GABA, we aimed to investigate the reliability of using either the co-edited GLX peak or the OFF subspectrum from GABA-edited data to quantify GLX. In 18 complete datasets, we show that for both GABA+ and MM-suppressed GABA acquisitions, the co-edited GLX peak is significantly correlated with GLX as measured from a SE-PRESS and is therefore a relatively reliable measure. Data were collected in a 27 ml voxel in the sensorimotor cortex. Different locations that are more susceptible to artifacts or poor shim need investigation to confirm this finding. Previous work [5], has suggested increasing the bandwidth of the editing pulses increases the co-edited GLX signal, which may further increase the reliability of using the co-edited GLX peak to reliably indicate GLX. Surprisingly, the GLX as measured from the OFF-subspectrum in GABA+ data was not correlated with the SE-PRESS GLX measures, indicating it is not a surrogate for GLX.Conclusion
The co-edited GLX peak from both GABA+ and MM-suppressed GABA acquisitions is a reliable measure of GLX, when using GLX measured from SE-PRESS data as a gold standard. The reliability of the GLX from the OFF-subspectrum from a GABA+ acquisition is not reliable.Acknowledgements
The authors thank the University of Calgary's University Research Grants Committee and NSERC i3T summer studentship program for funding .References
[1] Mullins et al. 2014 NeuroImage 86:43-52.
[2] Henry et al. 2001. Magn Reson Med 45:517–520
[3] Edden et al. 2012. Magn Reson Med 68:657–661
[4] Wilson et al. 2011. Magn Reson Med 65:1–12
[5] Snoussi et al. 2015. Proc ISMRM, p 4694
Figures