5623
Simultaneous measurement of metabolic rates of oxygen via 17O NMR imaging in brain and muscle tissue of rat at 16.4T1CMRR, Radiology, University of Minnesota Medical School, Minneapolis, MN, United States, 2MRC Department, MPI for Biological Cybernetics, Tübingen, Germany, 3Department of Physiology of Cognitive Processes, MPI for Biological Cybernetics, Tübingen, Germany, 4Radiology, University of Tübingen, Tübingen, Germany, 5MBIC, Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
In this study, we exploit the feasibility of the 17O MRSI technique for simultaneous measurement of the metabolic rates of oxygen in brain and surrounding muscle based on ROI analysis of dynamics of tissue H217O time courses acquired at 16.4T with 3D 17O MRSI. An established three-phase model originally developed for brain application was extended with certain assumptions applied to the resting temporalis muscle of rats.
Introduction
The measurement of cellular oxygen metabolism via inhalation of 17O2 through ultra-high field 17O MRSI is a promising tool for brain research and neuroscience 1. The imaging technique has been successfully employed to measure the cerebral metabolic rate of oxygen (CMRO2) in brain and the heart metabolic rate of oxygen: two organs with high aerobic metabolism 2. However, it hasn’t been tested and demonstrated if the same 17O technique is valid to be applied to the muscle with the extremely low activity of oxygen metabolism at the resting state 3.
In this study, we exploit the feasibility of the 17O MRSI technique for simultaneous measurement of the metabolic rates of oxygen in brain and surrounding muscle based on ROI analysis of dynamics of tissue 17O water time courses acquired at 16.4T with 3D 17O MRSI. An established three-phase model originally developed for brain application 4 was extended with certain assumptions applied to the resting temporalis muscle of rats.
Materials and Methods
Animal preparation, physiological monitoring
Five male Wistar rats were intubated for ventilation under anesthesia maintained through i.v. infusion of alpha-chloralose at 50-70mg/kg/h. Ventilation was set to a respiration rate of 70/min and arterial blood gas samples were drawn from the tail artery 5 to adjust ventilation volume to maintain pCO2 ~ 30-35 mmHg, pO2 > 100 mmHg and pH~7.4. Inhalations of enriched (~70% 17O2) gas were performed for 15 min each during a total MRSI acquisition of 54 min (109 volumes with 30s per 3D CSI-FSW, TR 5ms, TE 0.5ms, FOV 27.5x12.5x18 mm3/ Matrix 9x4x4, nominal ZF2 30x14x14, FA 68°). Afterwards post-mortem scans were performed in the same position (TA>6h) at very high resolution for coregistration 6.
MRI setup
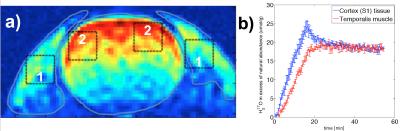
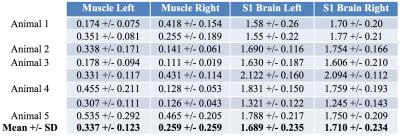
Imaging was performed using a custom-built 17O quadrature/1H butterfly-loop coil at 16.4T. 17O CSI intensities were normalized to a natural abundance water content of 0.037% by assuming similar water content for both brain and muscle. Inhalation time courses and washout of H217O were fitted using a three phase model for metabolism and circulation parameters 4. ROI averages of 3x3 voxels were outlined in the post-mortem images for muscle and brain tissue in the somatosensory cortex (S1) (Fig. 1a), corresponding time courses (Fig 1b) and single voxel estimates were pooled (Table 1). All selected voxels were relatively close to the highest sensitivity of the surface coil.
Results
The time courses of the two different tissue types represented very different dynamics during the inhalation phase and converged to the same level after the much longer equilibrium phase (Figure 1b). The averaged metabolic rates of oxygen (n=5) were found to be 1.70 and 0.30 μmol/g/min for brain and muscle, respectively.Discussion and Conclusion
In this study we showed time courses in low metabolizing muscle tissue and contrasted the dynamics against brain CMRO2. The results confirmed a large difference (> 5 times) in oxidative metabolic activity between the two types of tissues. In the perspective of technology, this study clearly indicates the feasibility and sensitivity for noninvasively detecting both high (brain) and low (muscle) rates of oxygen metabolism in vivo. It should be useful for understanding the aerobic metabolism in healthy and diseased condition.Acknowledgements
The study was funded by the Max Planck Society and NIH Grants: RO1 NS70839, MH111447; R24 MH106049, P41 EB015894 and P30 NS076408.References
1. Zhu, X.-H. & Chen, W. In vivo oxygen-17 NMR for imaging brain oxygen metabolism at high field. Prog. Nucl. Magn. Reson. Spectrosc. 59, 319–335 (2011).
2. Stanley, W. C., Recchia, F. A. & Lopaschuk, G. D. Myocardial substrate metabolism in the normal and failing heart. Physiol. Rev. 85, 1093–1129 (2005).
3. Basal Metabolic Rate in man. (1981). http://www.fao.org/3/contents/3079f916-ceb8-591d-90da-02738d5b0739/M2845E00.HTM Accessed November 9, 2016.
4. Atkinson, I. C. & Thulborn, K. R. Feasibility of mapping the tissue mass corrected bioscale of cerebral metabolic rate of oxygen consumption using 17-oxygen and 23-sodium MR imaging in a human brain at 9.4 T. NeuroImage 51, 723–733 (2010).
5. Balla, D. Z., Schwarz, S., Wiesner, H. M., Hennige, A. M. & Pohmann, R. Monitoring the stress-level of rats with different types of anesthesia: A tail-artery cannulation protocol. J. Pharmacol. Toxicol. Methods 70, 35–39 (2014).
6. Wiesner, H. M. et al. 17O relaxation times in the rat brain at 16.4 tesla. Magn. Reson. Med. 75, 1886–1893 (2016).
Figures