5621
Feasibility of localized, 1H decoupled natural abundance 13C-MRS of human brain at 7T using double tuned array coils and polarization transfer1Laboratory for Functional and Metabolic Imaging, CIBM, EPFL, Lausanne, Switzerland, 2Department of Radiology, Universities of Lausanne and Geneva, Lausanne, Geneva, Switzerland
Synopsis
The inherent low sensitivity of 13C-MRS makes detection of natural abundance metabolites in human brain challenging. We aimed to demonstrate that double-tuned array coils are particularly well-suited for 13C-MRS studies in vivo, as they provide high sensitivity and high transmit efficiency over a large FOV. To further enhance the SNR, DEPT sequence was used to transfer polarization from 1H to 13C, as well as WALTZ-16 1H-decoupling, all within FDA guidelines. Natural abundance metabolites such as glutamate, glutamine, NAA and creatine were successfully detected and decoupled in 30 minutes acquisition time, showing strong efficiency and sensitivity of our measurement setup.
Introduction
13C-MRS is a powerful non-invasive technique to assess human metabolism. However, the low inherent sensitivity of 13C-MRS remains the main drawback for human studies. To increase the SNR of natural abundance 13C-MRS, radio-frequency coils and pulse sequences have to be extremely efficient. Recently, double tuned arrays of surface coils were proposed to detect more efficiently 13C signals than linear coil designs, offering extended field of view and higher transmit efficiency at both 1H and 13C frequencies.1,2 In this study, we proposed to use double tuned array coils for 13C-MRS studies in human brain at natural abundance at 7T. We also investigated on DEPT sequence performance for 1H to 13C polarization transfer using array coils at ultra-high field, in order to increase the SNR.3 Finally, we intended to employ efficient 1H decoupling in respect to FDA guidelines to fully decouple the 13C resonances.
Methods
Coil design: 13C-MRS was performed using a double tuned phased array coil1 that can be used either for brain or muscle studies2 (fig.1). The coil is composed of a 4-channel 13C array (88x80mm2 loops) superimposed by a 4-channel 1H array (120x90mm2 loops). Its extended FOV covers the posterior half of human brain with high sensitivity at 13C frequency. The coil exhibits high transmit efficiency at 1H frequency and strong isolation between 1H and 13C channels, which is mandatory for 1H decoupling to be applied during 13C acquisition. Pulse sequence: To further improve sensitivity of 13C detection, polarization was transferred from 1H to 13C using a semi-adiabatic version of DEPT sequence3,4, adapted for single-channel systems5 (fig.2). Signal localization was performed on 1H spins using 3D-ISIS. The 13C excitation was a segmented 0o BIR-4. Spoiler gradients between the radiofrequency pulses were added to eliminate unwanted coherences. Broadband 1H decoupling (WALTZ-16)6 was applied during 13C signal acquisition. To evaluate the polarization transfer efficiency, this sequence was compared to direct 13C excitation (3D-ISIS + 90o BIR-4 at 13C frequency). MR measurements: MR experiments were performed on a 7T human scanner (Siemens, Erlangen, Germany). First and second order shims were adjusted using FASTMAP7. Glucose C1 resonances from a 6x6x6cm3 VOI of a 4L phantom were acquired using direct excitation (6ms 90o BIR-4, δ13C=96.6ppm, TR=5s, 8avg.) and DEPT (J=150Hz, δ13C=96.6ppm, δ1H=4.6ppm, αy=90o (optimal for CH detection), TR=5s, 8avg.). MR spectra from 13C isotopes’ natural abundance were acquired on a healthy female volunteer (26 years old, 63kg) who gave informed consent according to the procedure approved by the local ethics committee. The head of the volunteer in supine position was placed on top of the coil. 13C MR signals from a 245mL VOI in parietal and occipital lobes were acquired with DEPT sequence (J=120Hz, δ13C=34.2ppm, δ1H=2.3ppm, αy=45o (optimal for CH2 detection), TR=5s, acquisition duration=102ms, Acq. time=30min.). WALTZ-16 was applied during 52ms. SAR was maintained within FDA guidelines. Spectra from each coil were combined using WSVD method.8Results and discussion
The 3.4 times SNR enhancement of Glucose C1 resonances while using DEPT compared to direct detection was close to theoretical maximum value of $$$\frac{γ_{1H}}{γ_{13C}}$$$=4 (fig.3a). The two doublets were also successfully merged into two singlets when WALTZ-16 was used during signal acquisition (fig.3b). These results demonstrate that 1H excitation is relatively homogeneous over the large 13C FOV and that polarization transfer techniques can be applied with a double tuned array coil. 13C natural abundance cerebral metabolites, such as glutamate (Glu C3 and C4), glutamine (Gln C4), N-acetylaspartate (NAA C3 and C6), creatine (Cr C2), β-Hydroxybutyrate (β-HB C2 and C4) and taurine (Tau C1), were successfully detected in the brain in 30 minutes (fig.4). These resonances were effectively decoupled. β-Hydroxybutyrate was present in brain as a result of the low-carbohydrate diet of the volunteer.
Conclusion
It is feasible to perform in vivo natural abundance 13C-MRS in human brain at 7T using double tuned array coils. The gain of sensitivity provided by these coils, the ability to transfer polarization from 1H to 13C and to apply 1H decoupling during signal acquisition is a result of their high transmit efficiency, relatively homogeneous 1H excitation field and strong isolation between 1H and 13C channels. Consequently, polarization transfer and 1H decoupling can be used in vivo within FDA guidelines, allowing easy detection of natural abundance brain metabolites while maintaining the total acquisition time short. These preliminary results demonstrate that double-tuned array coils are particularly well-suited for 13C-MRS at ultra-high field.
Acknowledgements
Supported by Centre d’Imagerie BioMédicale (CIBM) of the UNIL, UNIGE, HUG, CHUV, EPFL and the Leenaards and Jeantet Foundations.References
1. Donati G et al. 8-Channel Double Tuned 13C-1H Transceiver Phased Array for 13C MRS in Human Brain at 7T. Proceedings of ISMRM 23rd Meeting (Toronto), 2015.
2. Donati G et al. 13C MRS in human calf muscles at 7T using a double tuned 4 channel 13C-4 channel 1H transceiver phased array. Proceedings of ISMRM 24th Meeting (Singapore), 2016.
3. Doddrell DM et al. Distortionless enhancement of NMR signals by polarization transfer. Journal of Magnetic Resonance. 1982 Jun 15;48(2):323-7.
4. Henry PG et al. 1H-localized broadband 13C NMR spectroscopy of the rat brain in vivo at 9.4 T. Magnetic resonance in medicine. 2003 Oct 1;50(4):684-92.
5. Klomp DW et al. Polarization transfer for sensitivity-enhanced MRS using a single radio frequency transmit channel. NMR in biomedicine. 2008 Jun 1;21(5):444-52.
6. Shaka AJ et al. Evaluation of a new broadband decoupling sequence: WALTZ-16. Journal of Magnetic Resonance. 1983 Jun 15;53(2):313-40.
7. Gruetter R. Automatic, localized in vivo adjustment of all first-and second-order shim coils. Magnetic Resonance in Medicine. 1993 Jun 1;29(6):804-11.
8. Rodgers CT et al.
Receive array magnetic resonance spectroscopy: whitened singular value
decomposition (WSVD) gives optimal Bayesian solution. Magnetic resonance
in medicine. 2010 Apr 1;63(4):881-91.
Figures
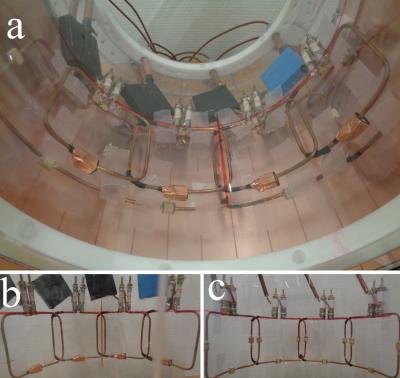
Figure 1: (a) Double-tuned transceiver phased-array, concentric combination of (b) a 4 channel 13C array (∅=23cm, loops size: 88x80mm2) and (c) a 4 channel 1H array (∅=26cm, loops size: 120x90mm2).1,2
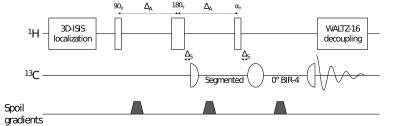
Figure 2: Overview of 1H-localized DEPT sequence (ΔS=45μs, ΔA=(1/2J)+ΔS+d90o, αy=90o to optimally detect CH, 45o for CH2 and 30o for CH3) used to transfer polarization from 1H to 13C, leading to a theoretical maximum increase in SNR of γ1H/γ13C=4).3-5
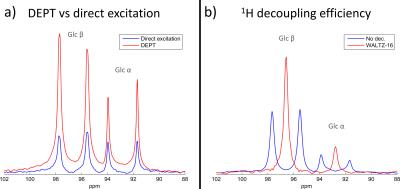
Figure 3: Glucose C1 resonances from a 6x6x6cm3 VOI in 4L phantom measured with the double-tuned array (TR=5s, 8avg.) :
a) 3.4 times increased SNR with DEPT (J=150Hz, δ13C=96.6ppm, δ1H=4.6ppm, αy=90o) compared to direct 13C detection (6ms 90o BIR-4, δ13C=96.6ppm).
b) WALTZ-16 1H-decoupling applied during 13C DEPT signal acquisition merges successfully both doublets into singlets.

Figure 4: In vivo 13C-MRS of natural abundance metabolites in human brain acquired in 30 minutes in a 245mL VOI in parietal and occipital lobes. The high sensitivity, as a result of the efficiency of the double tuned array coil, the 1H to 13C polarization transfer using DEPT sequence and the 1H decoupling applied during signal acquisition allows easy detection of several metabolites in a short acquisition time, within the FDA guidelines for SAR.