5616
Short TE PRESS-based proton observed carbon edited (POCE) 13C Magnetic Resonance Spectroscopy with a volumetric 1H transmitter for in vivo rat brain imaging at 7T1Biomedical Engineering, McGill University, Montreal, QC, Canada, 2Brain Imaging Centre, Douglas Mental Health University Institute, Montreal, QC, Canada, 3Department of Psychiatry, McGill University, Montreal, QC, Canada, 4Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States
Synopsis
Carbon-13 (13C) magnetic resonance spectroscopy (MRS) remains to be the only noninvasive method capable of measuring neuroenergetics and neurotransmitter cycling in the brain in vivo [1]. However, 13C MRS is a challenging technique to implement, and suffers from low sensitivity. In this study we investigated a short TE (12.6 ms) PRESS localized proton observed carbon edited 13C MRS utilizing a volumetric resonator for proton excitation. The designed platform demonstrates high sensitivity to 1H signals, provides excellent localization, and high resolution 1H and 1H-[13C] spectra for in vivo rat brain imaging.
Purpose
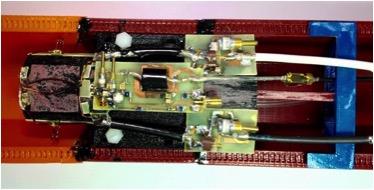
In this study a 1H-[13C] surface coil, similar to previous descriptions [2], is designed with the addition of a detunable 86 mm volumetric resonator for proton excitation, thus averting the requirement for adiabatic localization. The 1H-[13C] surface coil (Figure. 1) consists of a 1H Receive (Rx) - only surface coil, and a quadrature driven orthogonally placed pair of surface coils for editing pulses and heteronuclear decoupling on the 13C frequency. The aim was to develop a coil platform that would enable the realization of a short TE PRESS-localized POCE sequence (PRESS-POCE) utilizing short conventional pulses on the 1H channel. Furthermore, by incorporating a dedicated 1H Rx-only coil, the requirement for a T/R switch before the low noise 1H preamplifier is nullified; a highpass filter and preamplifier can be placed in close proximity to the coil, thus minimizing SNR losses inherent with POCE MRS systems described in the literature.Methods
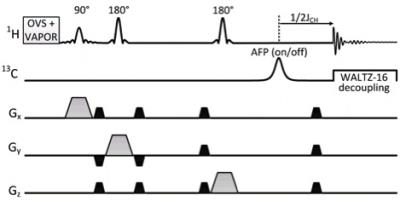
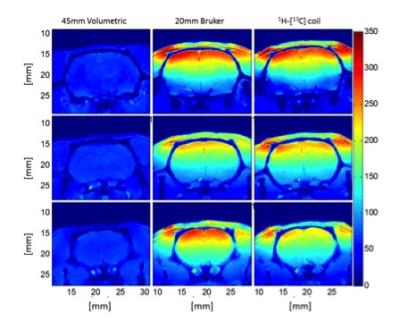
A short TE (12.6 ms) PRESS-POCE sequence was implemented as illustrated in Figure 2. The spoiler gradient duration (illustrated with black trapezoids in Figure. 2) was reduced to 0.35 ms and the gradient strength was set to 80% of max value which provided a balance between short TE and excellent voxel localization. The 13C editing pulse was placed 1/2JHC ms before the final echo utilizing AFP sech inversion. WALTZ-16 was employed for heteronuclear decoupling with each composite pulse segment empirically determined to be 0.7 ms in length to provide adequate decoupling (>70% decoupling efficiency) for > 40ppm of the 13C spectrum bandwidth on a 6 x 6 x 6 mm3 voxel. Sensitivity of the 1H Rx-only element of the 1H -[13C] coil was compared against an equivalent geometry (20 mm) Bruker Rx-only surface coil with integrated preamplifier (T115534; Bruker, Billerica, MA, USA), and a rat brain optimized 45 mm Transmit/Receive birdcage coil (Figure. 3). Two male Long Evans (Charles River Laboratories, Montreal, QC, Canada) rats weighing 260-300 g were used for this study. Following an overnight fast of 14 hours, the tail vein on one animal was cannulated for the infusion of 1-13C Glucose, under free-breathing of 2% isoflurane anesthesia. The animal was maintained under anesthesia and placed in the MRI scanner with the 1H-[13C] coil setup. All experiments were performed on a Bruker (Billerica, MA, USA) Biospec 70/30 horizontal bore preclinical scanner with an actively shielded gradient insert (120 mm inner diameter, 650 mT/m in 150 μs). Animal preparation and procedures were previously approved by McGill University’s animal research committee and are in accordance with guidelines set by the Council on Animal Care. An infusion of 1.1M 1-13C glucose (99.9% enriched Cambridge Isotope Laboratories Inc., Andover, MA, USA) in saline was prepared and delivered intravenously via tail vein 8.5 minutes after the start of the PRESS-POCE sequence (each 8.5 min block with 128 averages, TR = 4 s). A bolus of 350 μl was infused over the first 15 seconds (5400 μl/kg/min), and the infusion rate was tapered off exponentially every 30 seconds to rapidly raise and maintains plasma glucose levels at ~15 mM.Results
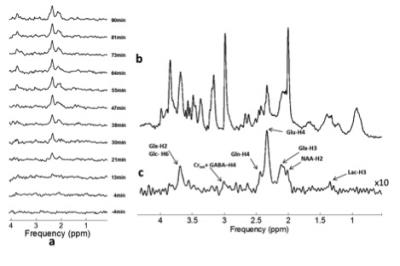
The 1H-Rx-only element of the 1H-[13C] coil shows comparable sensitivity profiles to the Bruker-20 mm surface coil (Figure 3). Both surface coils demonstrate superior SNR up to depths of ~8 mm relative to the 45 mm volumetric coil; thus providing SNR benefits for studies where spectroscopy voxels are placed proximal to the coil. Excellent Localization performance was obtained for the POCE sequence with a mean signal value < 0.3% for all directions outside the selected region. Figure 4 summarizes 1H and 1H-[13C] spectra obtained from a 100 μl voxel primarily encompassing the anterior cerebral cortex. Figure.10a illustrates a time series of 1H-[13C] spectra, with each individual spectrum arising from an 8.5 min acquisition (128 repetitions, TR=4 s), repeated for 94 mins, initiated 8.5 mins before the onset of the 1-13C Glucose infusion. The Glu-H4, Glx-H3 peaks and the Glx-H2 peak are visible approximately 13 min and 21min following the infusion, respectively. The edit-OFF spectra in Figure. 4b demonstrate excellent spectral resolution comparable to spectra at 7T reported elsewhere [3].Conclusion
A platform for 13C POCE MRS using PRESS-based localization is described. The PRESS sequence is readily available with most MRI scanners, thus the implementation of a short TE PRESS-POCE sequence is relatively straightforward (provided a compatible 1H -[13C] coil is used), compared to a LASER- or ISIS-based implementation. The feasibility of performing dynamic 13C POCE MRS in vivo is validated by POCE spectra obtained during the infusion of 1-13C glucose.Acknowledgements
This research was funded by NSERC (Grant No. RGPIN-2014-07072) awarded to JN, FRSQ Subvention pour projet de développement stratégique innovants 2012-2016 (Grant No. 26679) awarded to MR and QBIN travel grant awarded to CK. The authors thank Dr. Doug Rothman and Dr. Robin de Graaf for stimulating scientific discussions on 13C experiments and invitation to visit Yale University. The authors thank Pier Larochelle for her assistance with animal preparations for 13C experiments. The authors thank Saaussan Madi for Bruker related support, Helmut Stark, and Steven Toddes for their insights on related RF Engineering. Finally the authors thank the Robarts Institute of Western University for hosting and accepting CK for a high Field MRI winter school workshop (2015), and the McKnight Brain Institute at University of Florida for hosting an RF coil workshop (2016).References
1. Rothman D et al. NMR Biomed, 2011;24(8):943-957.
2. Adriany G et al. MRM, 1997;125(1):178-184.
3. de Graaf R et al. MRM, 2003;49(1):37-46.
Figures