5615
Improvement in 31P CSI voxel tissue segmentation1Psychiatry, University of Utah, Salt Lake City, UT, United States, 2The Brain Institute, University of Utah, Salt Lake City, UT, United States
Synopsis
The aim of the present study was to test a novel method for improving the subcortical tissue segmentation results, of the anatomical brain images acquired using a 31P/1H dual-tuned coil. When a dual-tuned 31P/1H coil is utilized to perform phosphorus-31 magnetic resonance spectroscopy studies of subcortical brain regions, the resulting anatomical images suffer from both low signal-to-noise ratio, and from reduced image contrast. By registering this volume image on a second anatomical image acquired using a single-tuned, 12 channel 1H head coil, we found that the subcortical tissue segmentation accuracy was significantly improved.
Introduction:
High energy phosphates (HEP) including phosphocreatine (PCr) and nucleoside triphosphate (NTP) play a critical role in neuronal activity. Tissue-specific changes in HEP are associated with abnormal brain function, and can be measured with 31P NMR spectroscopy. However, detection of low-concentration HEP requires large voxel sizes, limiting the ability to interrogate smaller brain structures. To measure HEP, chemical shift imaging (CSI) is preferred if data can be acquired using a 31P/1H dual-tuned volume coil, which is designed to optimize 31P signal detection. When the spectral signal is localized, anatomical images must be acquired along with the 31P spectrum from the same tissue location. Due to the high concentration of water in brain relative to HEP, suboptimal single-channel 1H coil sensitivity within 31P/1H dual-tuned coils is common, reducing structural image quality. When the volume of interest is subcortical, the dual-tuned coil’s signal-to-noise ratio (SNR) and image contrast are reduced even further. The reduced contrast and large voxel introduce another challenge to studying subcortical structures: the need to correct for partial volume artifacts. Recent work has measured functional brain activity, 1H, and 31P metabolites simultaneously. However, to achieve an optimal 1H functional image/proton-1 MRS signal, a high-performance 1H coil is needed. Using two coils requires repositioning subjects in the scanner, but is acceptable depending on the resulting improvements in data quality. For this study, we tested a novel method that registers anatomical images acquired with a 31P/1H dual-tuned coil on those acquired with a 12-channel 1H coil, followed by application of inverse affine transformation, the results of which provide a high-contrast image for 3D CSI voxel tissue segmentation.Method:
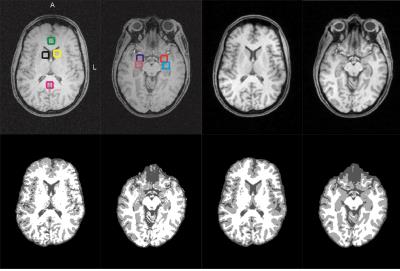
We enrolled n=25 healthy subjects (age=39.9±13.5). The protocol was approved by the University of Utah Institutional Review Board, and written informed consent was obtained from all subjects. Scans were performed using a 3 Tesla (T) clinical MRI system. A 3D CSI pulse sequence was implemented to acquire spectral data. The first T1 weighted volume image was collected using a 31P/1H dual-tuned coil. Following 31P data acquisition, a 12-channel 1H volume coil was used to acquire a second anatomical image. When two T1-weighted images are obtained from one subject, affine transformation can be used to co-register the images. The images were extracted with the FSL BET tool [1]. The FSL FLIRT was then used to register the image acquired with the 31P/1H coil into the 1H head coil image, and the affine transformation matrix was derived, converting the high-contrast T1-weighted image to phosphorus data native space. To perform tissue-specific data analysis, and minimize CSI array misalignment, the CSI grid was aligned according to the property of CSI grid re-shifting with an additional linear phase application in the k-space domain. The CSI voxel center coordinate was defined in the MNI space. This center coordinate was mapped from the MNI space to the phosphorus data native space. The transformation matrix between the standard image in the MNI space and the registered T1-weighted image in subject native space was computed using the FSL FLIRT and FNIRT tools. Both T1-weighted images were segmented using the FSL FAST tool [2]. To demonstrate differences in tissue segmentation between the original T1-weighted image and the registered T1-weighted image, we selected several gray-matter-rich regions (Fig. 1), such as anterior cingulate cortex (ACC), posterior cingulate cortex (POC), left/right amygdala (AMY_L/AMY_R), left/right caudate nucleus (CAU_L/CAU_R), and left/right hippocampus (HIP_L/HIP_R).Results & Discussions:
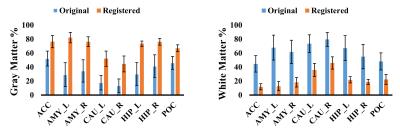
Fig.2 shows the percentage of gray matter (GM) and white matter (WM) within the specified brain voxels. We found that the GM content of subcortical regions was underestimated, when only the 31P/1H dual-tuned coil T1 image is segmented. Thus, application of registered T1-weighted images significantly improved detection of subcortical GM. Furthermore, the standard deviation in the registered segmentation images is smaller than that of the original T1-weighted image, across subjects. Two factors may contribute to this improved segmentation, and reduced measurement variability. First, greater contrast in the registered T1-weighted image likely improves the accuracy of FSL FAST’s segmentation. Second, the registered image’s increased SNR provides better affine transformation from the T1-weighted image in subject native space to standard reference image in the MNI space. The previous operation yields consistent voxel placement at the desired voxel location. This improved voxel placement should be useful for both longitudinal and multisite studies. Further, these improved segmentation results will enable investigators to more accurately correct partial volume effects, despite the large voxel sizes required for 31P research.Acknowledgements
No acknowledgement found.References
[1] Smith, S.M., Fast robust automated brain extraction. Hum Brain Mapp, 2002. 17(3): p. 143-55.
[2] Zhang, Y., M. Brady, and S. Smith, Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging, 2001. 20(1): p. 45-57.
Figures