5610
Effect of hyperbaric oxygenation on human brain phosphate metabolites at 3 Tesla. In vivo 31P MRS study1N.M.Emanuel Institute of Biochemical Physics of RAS, Moscow, Russian Federation, 2Radiology, Research Institute for Childen Emergency Surgery and Traumatology, Moscow, Russian Federation, 3National Research Nuclear University "MEPhI", Moscow, Russian Federation, 4Hyperbaric Oxygenation Dept., Research Institute for Childen Emergency Surgery and Traumatology, Moscow, Russian Federation, 5N.N. Semenov Institute of Chemical Physics of RAS, Moscow, Russian Federation
Synopsis
This study is aimed to reveal the effects of hyperbaric oxygenation on human brain phosphate metabolites using 31P MRS. At first, 31P MRS study was conducted, after that the subjects took a session in hyperbaric chamber (1.2 atmosphere, 100% O2) and then 31P MRS study was repeated. The increase of α-ATP peak intensity was revealed after hyperbaric oxygenation, while other peak intensities and pH remained unchanged. This phenomenon is likely to happen because of [NAD(H)] increase, that might confirm the positive effect of hyperbaric oxygenation on human brain metabolism.
Introduction
Hypoxia causes disorders in brain energy metabolism. One of perspective ways of treatment having an anti-hypoxic effect is hyperbaric oxygenation (HBO). There are clinical and laboratory studies that have demonstrated the effectiveness of HBO in different cases, for example, after CO poisoning1. The in vivo studies of HBO influence on energy metabolism might be of interest, and 31P MR spectroscopy is a unique noninvasive method for it. The aim of this study is to reveal the effect of one low-pressure (1.2 atmosphere absolute, ATA) HBO session on 31P MRS metabolites of the normal human brain.Materials and methods
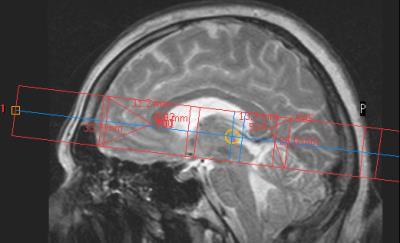
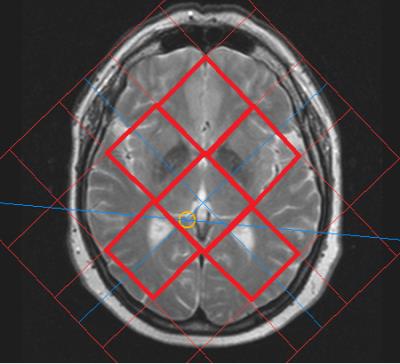
Eleven healthy subjects with no contraindications to MR methods and HBO became the participants of this study. Each subject has passed diagnostic MRI (T1-, T2-weighted, FLAIR, DTI) that revealed no abnormalities. Philips Achieva 3.0T, 31P/1H Dual bird-cage coil by Rapid Biomed and hyperbaric chamber Sechrist 3200 were used. The plan of the study is as follows: 1) in MRI scanner survey images (30 seconds), axial (1 minute), sagittal (1 minute) T2-weighted images and 2D 31P MRS (10 minutes) data were obtained; 2) the subject proceeded to the hyperbaric chamber for 50 minutes (5 minutes for achieving constant 1.2 ATA mode, 40 minutes at the mode and 5 minutes for decompression); after that step 1) was repeated. The axial and sagittal images were used for 2D 31P MRS voxels location, as it is demonstrated on figures 1 and 2. The match of spectroscopy voxels location before and after HBO session was provided by placing the spectroscopy slice symmetrically in relation to fissura longitudinalis cerebri on axial images, and by keeping the same distances from genu and splenium of corpus callosum to several spectroscopy voxels (see figure 1). The parameters of 2D 31P MRS: slice thickness = 30 mm, FOV = 200x200 mm, voxel size = 40x40 mm. Spectroscopy data was obtained with ISIS pulse sequence (TE = 0.3 ms, TR = 1900 ms, NSA = 16, FA = 35°, broadband NOE (mix time = 1560 ms) and decoupling).Data processing
The 2D 31P data obtained preprocessing and quantification was performed in jMRUI 5.2 program. The FIDs of voxels that were completely located in the brain of a subject (shown on fig.2 with red frames) were averaged, two spectra remained for each subject: before and after HBO session. The integral intensities of spectral peaks were quantified using AMARES algorithm. For each subject in spectra before and after HBO session the intensities of individual peaks were normalized to the sum of integral intensities of all peaks (total 31P). The pH value before and after HBO session was calculated from PCr-Pi chemical shift difference. The statistical processing was performed in STATISTICA 12 program using Mann-Whitney U-criterion.Results
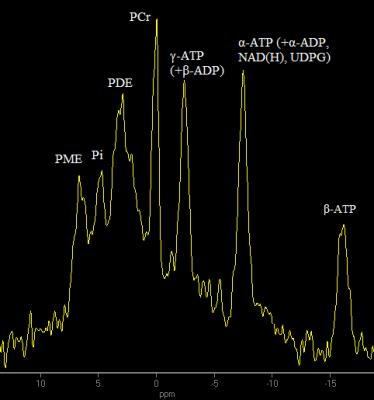
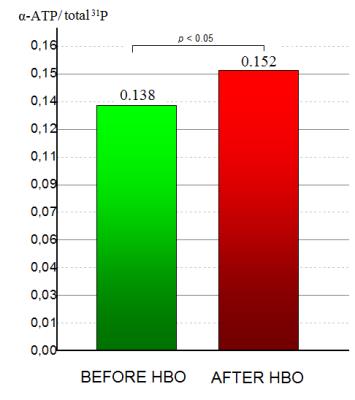
The typical spectrum obtained in this study is demonstrated on figure 3. After HBO session there was a statistically significant (p<0.05) increase of α-ATP peak integral intensity relative to total 31P (see figure 4). No changes in other peak integral intensities relative to total 31P were revealed. HBO also had no effect on pH value.Discussion
The revealed increase of α-ATP peak integral intensity while β-ATP remains unchanged testifies to the increase in intensities of signals that compose α-ATP peak besides ATP: α-ADP, nicotinamide adenine dinucleotide (NAD+ and NADH), uridine diphosphate glucose (UDPG). UDPG signal has two resonance frequencies: one of them is overlapped by an α-ATP peak, the second is at -9.72 ppm relative to PCr2. Since this peak cannot be picked out of noise neither in spectrum before, nor in spectrum after HBO session, we may conclude that UDPG does not make a substantial contribution to α-ATP change discovered in this study. Change in ADP concentration can also hardly be a reason of α-ATP intensity increase because no change in γ-ATP peak intensity (that is a superposition of γ-ATP and β-ADP) stimulated by HBO session is revealed. The only hypothesis remaining is that the effect is caused by [NAD+] and/or [NADH] increase. The HBO is known to change chemical equilibrium of [NAD+]/[NADH] to increase [NAD+]3, however, the spectral resolution at 3T does not allow to separately analyze NAD+ and NADH, and to detach them from α-ATP peak. The nature of [NAD(H)] increase phenomenon revealed in this study is a subject of future research. Nonetheless, since [NAD(H)] plays an important role in cell energy metabolism4, we suppose that the result obtained might be a confirmation of HBO effectiveness even at low pressures (p=1.2 ATA) in the context of energy metabolism stimulation in human brain.Acknowledgements
No acknowledgement found.References
1. G.G. Rogatsky, S. Meilin, N. Zarchin, S.R. Thom, A. Mayevsky. Hyperbaric oxygenation affects rat brain function after carbon monoxide exposure. UHM 2002, Vol 29, No. 1
2. Suzanne L. Wehrli, Michael J. Palmieri, Gerard T. Berry, Henry N. Kirkman, and Stanton Segal. 31P NMR Analysis of Red Blood Cell UDPGlucose and UDPGalactose: Comparison with HPLC and Enzymatic Methods. Analytical Biochemistry 202,105-110 (1992)
3. E. Meirovithz, J. Sonn, A. Mayevsky. Effect of hyperbaric oxygenation on brain hemodynamics, hemoglobin oxygenation and mitochondrial NADH. Brain research reviews, 54 (2007) 294 – 304
4. Berger F, Ramirez-Hernandez MH, Ziegler M. The new life of a centenarian: signalling functions of NAD(P). Trends Biochem Sci 2004;29:111–118.
Figures