5597
Metabolic differences in patients with Overt and Potential Celiac disease Studied by in-vitro Proton NMR1Nuclear Magnetic Resonance and MRI Facility, All India Institute of Medical Sciences, New Delhi, India, 2Pathology, All India Institute of Medical Sciences, New Delhi, India, 3Gastroenterology & Human Nutrition, All India Institute of Medical Sciences, New Delhi, India
Synopsis
Potential celiac disease (CeD) patients have immunological abnormalities similar to CeD but unlike CeD, their duodenum displays normal histology. In-vitro proton NMR study of small intestinal mucosa of these patients demonstrated metabolic abnormalities associated with the intestinal inflammation. Both potential CeD and CeD patients had lower concentration of histidine compared to controls while lower glycine was seen only in CeD. Since, both amino acids exert anti-inflammatory effects; their reduced levels suggested compromised cytoprotective mechanism. Significantly higher level of glycerophosphocholine seen in potential CeD compared to CeD might have contributed for renewal of enterocytes and thus to normal small intestine histology.
Introduction
Celiac Disease (CeD) is a common gluten-sensitive enteropathy characterized by complex interaction between genetic and environmental factors1. The wide spectrum of CeD range from a fully symptomatic form which is characterized by CeD specific serological markers [(anti- tissue transglutaminase (anti-tTG) antibody, antiendomysial antibody)] and small intestinal villous atrophy (overt) to asymptomatic forms, defined as latent and silent CeD. A unique category of subjects under gluten-sensitivity, defined as potential CeD are those that share similar immunological abnormalities as seen in CeD, however their small intestine is histopathologically normal. Bernini et al. documented that patients with potential CeD had similar sera metabolic abnormalities as seen in CeD when compared to controls and suggested that it might precede enteropathy2.Therefore potential CeD might be considered as model of an early stage disease in the development of CeD. Based on this hypothesis, present study investigated the metabolic profile of small intestinal biopsies from potential CeD and compared it with patients with CeD and disease controls (patients with dyspepsia) using in-vitro proton nuclear magnetic resonance (NMR) spectroscopy to gain an insight into underlying biochemical processes of CeD development.Patients and Methods
Sixty four patients with CeD (mean age 28.5 ± 11.4 yrs) and seven potential CeD patients (mean age 27.7 ± 12.5 yrs) were recruited. Thirty-five subjects (mean age 31.9 ±9.4 yrs) undergoing endoscopy for dyspepsia served as disease controls (referred as controls henceforth). An informed consent was taken and Institute Ethics committee approved the study. All patients were treated according to standard treatment regimen. Diagnosis of CeD was based on combination of clinical manifestations, CeD specific serological markers (anti-tTG antibody, antiendomysial antibody) and confirmed histological abnormalities using modified Marsh-Oberhuber classification following guidelines of European Society of Pediatric Gastroenterology Hepatology and Nutrition. During endoscopic examination, mucosal biopsies were obtained from the second part of duodenum (6 bits for NMR spectroscopy and 4 bits for histopathology). Perchloric acid extraction was used for extraction of water soluble metabolites. The lyophilized powder obtained was dissolved in deuterium oxide and sodium trimethyl silyl- (2,2,3,3-H4) propionate was added as a standard for chemical shift and quantification of metabolites. One and two dimensional total correlation spectroscopy NMR experiments were carried out at 700 MHz NMR spectrometer (Agilent, U.S.A.) and concentration of metabolites was determined. Mann Whitney (SPSS 20.0) test was used for comparison of metabolite levels in different groups and p-value <0.05 was considered significant.Results
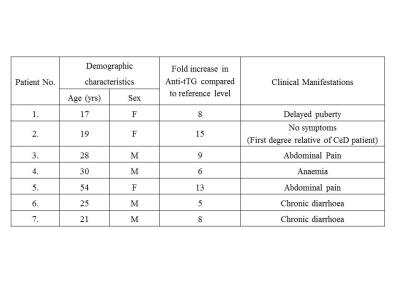
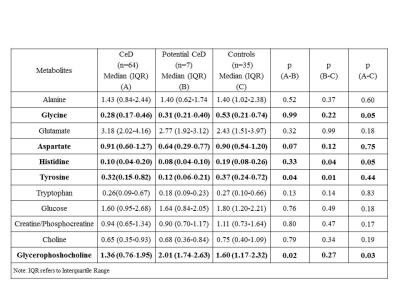
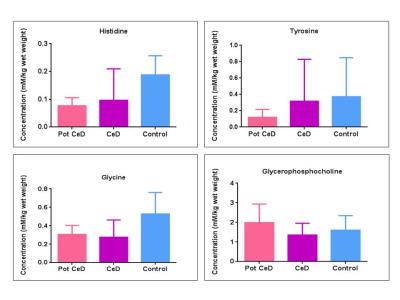
The demographic characteristics of patients with potential CeD presented in Table 1. The concentration of 11 metabolites including amino acids, membrane metabolites, metabolites related to energy metabolism are presented in Table 2. The level of histidine was significantly lower in potential CeD and CeD, while glycine was lower only in CeD compared to controls (Fig. 1, Table 2). The tyrosine level was lower in potential CeD than both CeD and controls. Glycerophosphocholine level was higher in potential CeD than CeD, however, it was similar to controls (Fig. 1).Discussion
To our knowledge, this is the first study profiling metabolic characteristics of small intestinal mucosa of patients with potential CeD in comparison to patients with CeD and controls. Our data showed significantly lower concentration of histidine in both potential CeD and CeD patients while lower level of glycine only in CeD in comparison to controls. Histidine is an essential amino acid which is being converted to histamine by histidine decarboxylase. Histamine then acts as an anti-inflammatory agent by suppressing intracellular signalling pathways that are involved in the production of several kinds of cytokines which lead to the intestinal inflammation3. Glycine has also been reported to provide several protective effects, including antiinflammatory, immunomodulatory and direct cytoprotective actions in intestine4. Thus, lower level of these amino acids might be attributed to their utilization for anti-inflammatory activity in small intestine. Level of aromatic amino acid tyrosine was lower in potential CeD compared to CeD and controls however, specific role of tyrosine in CeD remains to be investigated. Metabolic differences might also be related to the differences in gut microbiome5 in these categories of subjects. Potential CeD patients had significantly higher level of glycerophosphocholine which is a membrane component. Glycerophosphocholine is essential for the regulation of cell growth, differentiation and renewal of enterocytes. Hence its normal level maintained villous integrity in potential CeD while lower level in CeD patients was indicative of villous atrophy6.Conclusion
Distinct metabolic fingerprints of histopathologically normal intestinal mucosa of potential CeD in comparison to CeD and controls was seen. Results may have value in understanding biochemical processes underlying CeD development, however, validation in large cohort of patients needed.Acknowledgements
The Department of Biotechnology, Government of India is acknowledged for financial support (BT/Bio-CARe/01/233/2010-11).References
1. Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012; 18(42):6036-59.
2. Bernini P, Bertini I, Calabrò A et al. Are patients with potential celiac disease really potential? The answer of metabonomics. J Proteome Res. 2011 Feb 4;10(2):714-21. doi: 10.1021/pr100896s.
3. Son DO, Satsu H, Shimizu M. Histidine inhibits oxidative stress- and TNF-alpha-induced interleukin-8 secretion in intestinal epithelial cells. FEBS Lett. 2005; 579 (21):4671-7
4. Zhong Z, Wheeler MD, Li X, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6(2):229-40.
5. Zhang YJ, Li S, Gan RY, et al. Impacts of gut bacteria on human health and diseases. Int J Mol Sci. 2015; 16(4):7493-519.
6. Sharma U, Upadhyay D, Mewar S, et al. Metabolic abnormalities of gastrointestinal mucosa in celiac disease: An in vitro proton nuclear magnetic resonance spectroscopy study. J Gastroenterol Hepatol. 2015;30(10):1492-8.
Figures