5594
In vivo brain redox status and blood-brain barrier function in diethylmaleate-treated mice by EPR imaging and ME-MRIHirotada G Fujii1, Miho C Emoto1, Yuta Matsuoka2, and Ken-ichi Yamada2
1Sapporo Medical University, Sapporo, Japan, 2Kyushu University, Fukuoka, Japan
Synopsis
EPR imaging has been used to visualize redox status in oxidative brain diseases, but the role of cerebral glutathione (GSH) is not clear. In this study, using the mouse model of GSH depletion with diethylmaleate (DEM), the role of GSH in brain redox status was examined. The remarkable change in redox status in DEM-treated mouse brain was visualized with EPR imaging, and in vitro assay showed decrease in the level of GSH. ME-MRI clearly visualized blood-brain barrier dysfunction in DEM-treated mice. Results indicate that GSH plays an important role in the maintenance of both brain redox status and BBB integrity.
Puopose
Under normal physiological conditions, oxidative damage is prevented by the regulation of reactive oxygen species by the antioxidant network. Major antioxidants in brain are ascorbic acid (AsA) and glutathione (GSH), and the levels of these antioxidants change depending on the disease state. Nitroxides are redox-sensitive compounds and reduced in vivo in the presence of AsA and GSH. Therefore, electron paramagnetic resonance (EPR) imaging with nitroxides has been used to visualize the redox status in oxidative brain diseases (1), but the role of GSH on the reduction reaction of nitroxides is not clear. In the present study, using the mouse model of GSH depletion with diethylmaleate (DEM), the role of GSH in the brain redox status was examined non-invasively by EPR imaging with 3-methoxycarbonyl-PROXYL (MCP). Since GSH has a pivotal role in the maintenance of membrane integrity, it plays an important role in the proper functioning of blood-brain barrier (BBB). Therefore, the present study was also planned to see the effect of GSH depletion using DEM on BBB permeability by EPR imaging and ME-MRI.Methods
Paramagnetic nitroxide imaging probes: MCP was obtained from NARD Chemicals, Ltd. (Osaka, Japan). 3-Carboxyl-PROXYL (COP) was purchased from Sigma-Aldrich Co. (St.Louis, USA). DEM was obtained from Wako Pure Chemicals (Osaka, Japan). Animals: Male C57BL/6 mice aged 5 to 7 weeks with body weights of 20–25 g were used. DEM-treatment: Mice were given single injection of DEM (4mmol/kg), intraperitoneally. EPR images and ME-MRI of mice were carried out 2 h after DEM-treatment. MRI measurements: MRI of mouse heads was acquired using an MR mini scanner (MR Technology, Tsukuba, Japan) with a 0.5-T permanent magnet. ME-MRI was taken after administration of MnCl2 (12.5 mg/kg) to control and DEM-treated mice. EPR imaging measurements: All EPR images were acquired using an in-house built 750 MHz CW-EPR imager. Using rapid magnetic field scan system, the fastest data acquisition time for 3D-EPR images is about 9 sec in cases of 50 ms field scanning (6 mT field scan) and 181 projections.Results and Discussion
The distribution of a redox-sensitive nitroxide probe, MCP, in control and DEM-treated mouse heads was visualized with EPR images. The rate constant of the reduction reaction of MCP in mice was measured as an index of redox status in vivo. The pixel-based reduction rates of MCP in control and DEM-treated mouse heads were calculated from a series of temporal three-dimensional EPR images of mice. The obtained reduction rates at each pixel in the EPR images were displayed as a “redox map”. The redox map from control and DEM-tread mouse heads was co-registered to the anatomical image of mouse heads taken by MRI and was shown in Fig. 1. The co-registered images obtained from control and DEM-treated mouse clearly show that the remarkable difference in the reduction rates were found in brain area. Fig. 2A shows that the reduction rate of MCP in DEM-treated mouse brain was significantly lower than the value in control mice. The levels of several antioxidants (AsA and GSH) were measured in control and DEM-treated mouse brains. As shown in Fig.2B, the level of GSH in DEM-treated mouse brain was significantly lower than in control, although no difference was found in the level of AsA in both mouse brains. The results in Fig. 1 and 2 strongly indicate that the redox status in mouse brains is apparently changed by the level of GSH. Next, the effect of GSH depletion due to DEM treatment on BBB permeability was examined by MRI with gadolinium (Gd) and manganese (Mn) ions. Administration of DEM was found to increase the BBB permeability to Mn ions, but not to Gd ions. ME-MRI of control (-DEM) and DEM-treament mice were shown in Fig. 3. MRI results indicate observed BBB dysfunction was associated with brain GSH depletion.Conclusion
The remarkable change in redox status in DEM-treated mouse brain was visualized with EPR imaging. In vitro assay showed the decreased concentration of GSH, but not AsA, in brain after DEM treatment. ME-MRI clearly visualized BBB dysfunction in DEM-treated mice. These results indicate that cerebral GSH plays an important role in the maintenance of both brain redox status and BBB integrity.Acknowledgements
This work was supported by a grant from the Japanese Society for the Promotion of Science (24791318).References
1 Emoto CM, Fujii GH, et al. Dynamic changes in the distribution and time course of blood-brain barrier-permeable nitroxides in the mouse head with EPR imaging. Free Radic Biol Med. 2014; 74: 222-228.Figures
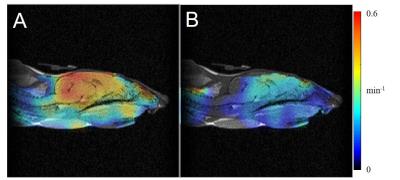
Co-registered images of redox map obtained by EPR imaging and anatomical image by MRI for control (A) and DEM-treated (B) mouse heads.
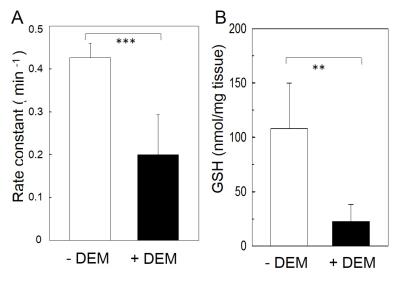
Reduction rates of MCP (A) and GSH level (B) in brain of control (-DEM) and DEM-treated (+DEM) mice. **: P << 0.01, ***: P << 0.001.
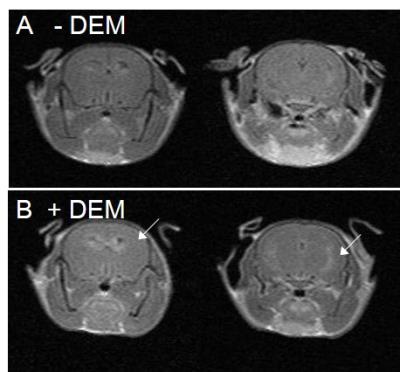
MEMRI of control (-DEM) and DEM-treated (+DEM) mice. The arrow indicates Mn2+ infusion.