5592
Characterization of Water Compartment Exchange in Ex-Vivo Human Cartilage Using Two-Dimensional Relaxometry1Magnetic Resonance Imaging and Spectroscopy Section, National Institute on Aging, Baltimore, MD, United States, 2University of California, Berkeley, CA, United States, 3Medstar Harbor Hospital, Baltimore, MD, United States
Synopsis
One-dimensional transverse relaxometry has proven to be an effective method for characterizing macromolecular compartments in cartilage. Two-dimensional studies extend the capabilities of these types of experiments, providing characterization of tissue compartments in terms of correlated relaxation times and providing a means of probing intercompartmental exchange. We provide results of T2-T2 and T1-T2 relaxometry experiments on human articular cartilage, indicating that exchange between tissue compartments may be augmented in degraded tissue.
Introduction
Osteoarthritis (OA) is a prevalent age-associated disease involving degeneration of cartilage extracellular matrix. Cartilage is composed of 65–80% water and 18–35% extracellular matrix by wet weight1, with the predominant matrix constituents being type II collagen and proteoglycan (PG). Bulk water, PG-associated, and collagen-associated water define compartments that can be identified using 1D magnetic resonance relaxometry (MRR) through evaluation of transverse signal decay1. 2D relaxometry expands the capabilities of MRR by characterizing water pools according to two parameters2, or evaluating exchange between pools3,4. Here, we investigate changes in extracellular compartmental exchange in human articular cartilage using T2-T2 and T1-T2 MRR2,3,4,5.Methods
Tissue collection: Cartilage from knee arthroplasty was obtained according to an IRB-approved protocol. Osteochondral plugs with 5 mm diameter and 5 mm height were harvested from the tibial plateau for immediate MRI assessment. Four samples were used. Samples 1, 2, and 3 were taken from non-load bearing regions and showed minimal visible degradation. Sample 4, from the load-bearing region, showed clear signs of degradation.
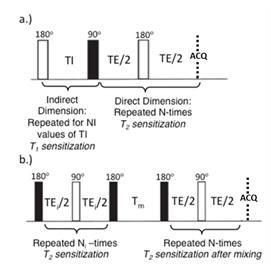
Magnetic resonance: Plugs were imaged in susceptibility-matched Fluorinert® FC-770 (Sigma–Aldrich, St. Louis, MO) at 4°C using a 9.4T Bruker Avance III NMR spectrometer with a Bruker Micro2.5 micro-imaging probe and a 5 mm solenoidal insert. Signal from the subchondral region and the cut sides of the sample were eliminated using saturation slabs. The surface normal to each sample’s articular surface was oriented at the magic angle. All 2D measurements were made using a non-localized CPMG readout with pulse length of 10 µs, echo time TE=100 µs, number of echoes NE=4096 and repetition time TR=10 s. T2-T2 experiments were acquired with 64 signal averages and 8 mixing times (Tm) of 10, 20, 40, 80, 160, 320, 480 and 640 ms. T1-T2 experiments were performed using an inversion-preparation with 16 logarithmically spaced inversion times. See Figure 1. 2D T2-T2 and T1-T2 plots were obtained through use of the 2D inverse Laplace transform6 implemented in MATLAB with regularization performed according to the discrepancy principle.
Results
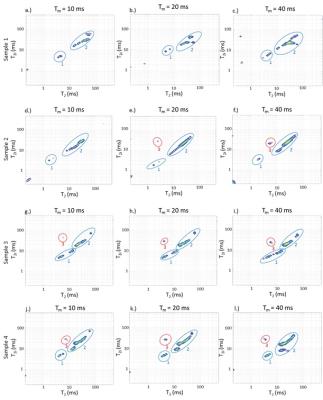
T2-T2 correlation spectra: Diagonal peaks indicate relaxation pools with T2 values indicated on the axes; off-diagonal peaks indicate exchange between pools. Exchange timescale is defined by the mixing times at which the off-diagonal peaks appear. While relatively unbound water and PG-bound water are expected to be the dominant signal components, 2D results feature a richer structure indicating heterogeneity in relaxation times within compartments.
Figure 2: T2-T2 distributions for mixing times of 10ms, 20ms, and 40ms. Components with T2 values ~50 - 100 ms are assigned to loosely bound water, while more rapidly relaxing components are assigned to macromolecular-bound water. Off-diagonal peaks show intercompartmental exchange. Visually non-degraded samples 2 and 3 show cross-peak development between these compartments on time scales of 10 - 20 ms. The third non-degraded sample, sample 1, did not exhibit intercompartment exchange over the maximum mixing time of 40 ms. Sample 4, the degraded sample, showed the most rapid development of exchange cross-peaks, which were well-established by 10 ms.
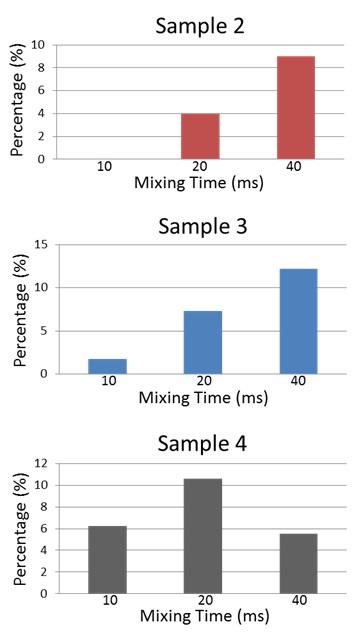
Figure 3: Relative off-diagonal cross peak areas, indicating buildup with mixing time followed by diminution for the case of most rapid exchange3.
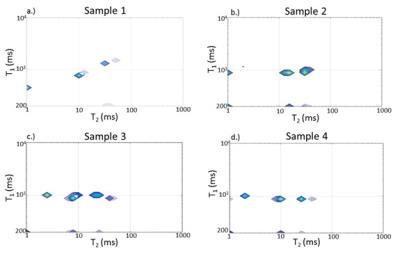
Figure 4: T1-T2 distributions, with components defined by joint (T1, T2) values. The small amplitude components at the edges represent inversion artifacts. In addition, for two of the non-degraded samples (samples 2 and 3) and for the degraded sample (sample 4), the T1 values of all components are equal, reflecting intercompartmental exchange rapid on the T1 time scale. For one of the non-degraded samples, sample 1, distinct T1 values are seen. This indicates intercompartment exchange which is slow on the T1 time scale, consistent with the lack of T2-T2 exchange peaks for this sample (Fig. 2).
Discussion
2D relaxometry was implemented in this work to characterize exchange between water compartments in human articular cartilage. T2-T2 experiments with variable mixing time define the time scale of exchange between water compartments3, while T1-T2 experiments show joint relaxation times for water compartments2. We found the most rapid exchange between relatively unbound water and macromolecular water in sample 4, the most degraded sample. This may be interpreted as loss of macromolecular integrity. In addition, one of the non-degraded samples, sample 1, displayed no exchange cross peaks in the T2-T2 experiment as well as distinct T1 values in the T1-T2 correlation spectrum, reflecting compartmental integrity. Correlative tissue histology is ongoing.Conclusion
2D MRR can characterize exchange between water compartments in human articular cartilage. This provides a marker of tissue integrity in healthy and degraded cartilage.Acknowledgements
No acknowledgement found.References
[1] Reiter DA, Roque RA, Lin PC, Doty SB, Pleshko N, Spencer RG. Improvedspecificity of cartilage matrix evaluation using multiexponential transverserelaxation analysis applied to pathomimetically degraded cartilage. NMR Biomed. 2011 Dec;24(10):1286-94.
[2] Warner J, Donell S, Wright K, Venturi L, Hills B. The characterisation of mammalian tissue with 2D relaxation methods. Magnetic Resonance Imaging. 2010 Sept; 28(7):971-981.
[3] Monteilhet L, Korb JP, Mitchell J, McDonald PJ. Observation of exchange of micropore water in cement pastes by two-dimensional T(2)-T(2) nuclear magnetic resonance relaxometry. Phys Rev E Stat Nonlin Soft Matter Phys. 2006.
[4] Fleury M, Soualem J. Quantitative analysis of diffusional pore coupling fromT2-store-T2 NMR experiments. J Colloid Interface Sci. 2009 Aug; 336(1):250-9.
[5] Dortch RD, Horch RA, Does MD. Development, simulation, and validation of NMRrelaxation-based exchange measurements. J Chem Phys. 2009 Oct; 131(16).
[6] Venkataramanan L, Song Y and Hurlimann MD. Solving Fredholm integrals of the first kind with tensor product structure in 2 and 2.5 dimensions. IEEE. 2002 May; 50(5):1017-1026.
Figures