5591
1H and 13C NMR evaluation of pH-dependent structural characteristics of lonidamine1University of Pennsylvania, Philadelphia, PA, United States, 2Thomas Jefferson University, Philadelphia, PA, United States
Synopsis
We seek to understand the pH-dependent physicochemical changes of lonidamine (LND), an antineoplastic drug, particularly to locate the sites of ionization. LND samples at pH 2, 7, and 13 were analyzed using 1H and 13C NMR. The results indicate that there is a noticeable change in the chemical shifts for a few atoms in LND from neutral to alkaline pH. These changes demonstrate that LND is ionized at its imidazole α-nitrogen. In addition, the expected ionization of the carboxyl group of LND at acid pH is not directly observed, and this may be due to a rapid-exchange phenomenon.
TARGET AUDIENCE
Investigators interested in antineoplastic drug, pH, ionization, 1H and 13C NMR, structural NMRINTRODUCTION
Lonidamine (LND), an antineoplastic drug, is a derivative of indazole-3-carboxylic acid. LND has limited antineoplastic activity as a single agent but has exceptional potential in modulating the activities of conventional chemotherapeutic agents,1-4 hyperthermia,5 radiation therapy,6 and photodynamic therapy.7 LND inhibits transmembrane monocarboxylate transporters (MCTs), the mitochondrial pyruvate carrier (MPC) and complex II of electron transport chain.1,8,9 Ionization of a molecule introduces a localized charge to its structure and thus affects the chemical shift of adjacent atoms. A variety of experimental methods using 1H and 13C NMR were employed to determine the location of the ionization event. In particular, we sought to delineate the pH-dependent physicochemical changes of LND by such NMR methods.METHODS
Lonidamine was dissolved in deuterated DMSO to a concentration of 50 mM. Titrations were performed by adding 1M HCl and 1M NaOH solutions and measuring an effective pH using a pH probe calibrated on aqueous solutions. LND solutions were titrated by addition of approximately 30 uL of aqueous solutions to a 500 uL sample of the DMSO-dissolved LND. Then, 0.5 ml portion of such solution was placed in a standard 5-mm o.d. NMR tube. 1H and 13C NMR spectra were recorded at 25ºC using a Varian 400 MHz spectrometer operating at 9.4 T, and equipped with temperature controllers. For 1H spectra, the chemical shift at 7.11 ppm corresponding to the proton on the 6-carbon of the dichlorobenzyl group was used as the reference point, since its chemical shift was predicted to be independent of pH. For 13C spectra, the chemical shift at 40.6 ppm corresponding to DMSO was used as the reference point, for the same reason. To record 13C NMR spectra, the standard measurement parameters were as follows: Repetition time (TR) = 1.5 s, pulse width (PW) = 6.2 μs, (pulse angle 45º), spectral width (SW) = 24.5 KHz, no. of acquisitions (N) = 1024. For recording 1H NMR spectra the following measurement parameters were used: TR = 8.5 S, PW = 9 μs (pulse angle 56º), SW = 6400 Hz, N = 32. The raw FID files were processed using NMR Utility Transform Software using the Fourier Transform and Phasing functions. The predicted spectra were obtained through the ChemNMR tool in ChemDraw.RESULTS
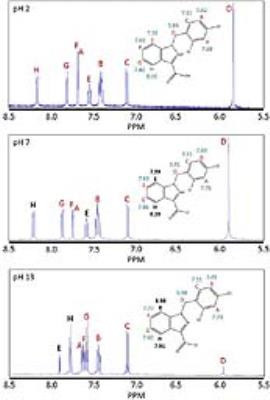
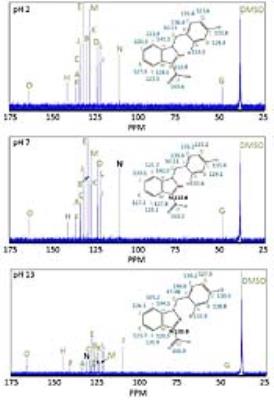
1H and 13C NMR indicate that there are noticeable changes in the chemical shifts of LND from acid to alkaline pH. The 1H NMR demonstrates greatest change in chemical shift between pH 7 and pH 13 at protons “E” and “H” (Labels provided in Figure 1). The changes in these chemical shifts were -0.99 ppm and 0.29 ppm, respectively (Table 1). In addition, the 13C NMR spectrum shows the greatest change in chemical shift between pH 7 and pH 13 at carbon “N” (Labels provided in Figure 2). The change in this chemical shift was -22.43 ppm (Table 1). Also, 1H and 13C NMR indicate that there are limited changes in the chemical shifts seen for LND from neutral to acid pH (Figures 1 and 2). All changes in chemical shift between pH 2 and pH 7 were sufficiently small compared to the changes in chemical shift between pH 2 and pH 13 (Table 1) to be neglected.DISCUSSION AND CONCLUSION
In aqueous solution, the solubility of LND dramatically decreases as the pH is lowered from 8.3 to 7.0. We hypothesize that this change in solubility results from protonation of the imidazole α-nitrogen of LND to produce a zwitterion, which rapidly crystalizes. Our results are in agreement with this hypothesis. In particular, the atoms which are closest to our proposed deprotonation site show the greatest change in chemical shift between pH 7 (neutral) and pH 13 (alkaline). Our 1H NMR demonstrates that the molecular environment changes most around protons “E” and “H”, while our 13C NMR demonstrates that the molecular environment changes most around carbon “N”. These two observations indicate that a substantial change must be occurring in the imidazole ring of LND from neutral to alkaline pH. The proposed deprotonation of the α-nitrogen would produce changes consistent with what we observed. The second pole of the proposed zwitterion, the carboxyl group, was not directly observed. This may be because there is rapid exchange occurring with the proton in the carboxylic acid, which would make the lifetime of the fully-protonated form of LND so short that NMR cannot detect it.Acknowledgements
Support of this project was provided by NIH grants RO1-CA129544 and RO1-CA172820.References
1. Nath K, Guo L, Nancolas B, Nelson DS, et al. Mechanism of antineoplastic activity of lonidamine. Biochim Biophys Acta. 2016;1866(2):151-62.
2. Nath K, Nelson DS, Heitjan DF, et al. Lonidamine induces intracellular tumor acidification and ATP depletion in breast, prostate and ovarian cancer xenografts and potentiates response to doxorubicin. NMR Biomed. 2015;28(3):281-90.
3. Nath K, Nelson DS, Ho AM, et al. 31P and 1H MRS of DB-1 melanoma xenografts: lonidamine selectively decreases tumor intracellular pH and energy status and sensitizes tumors to melphalan. NMR Biomed. 2013;26(1):98-105. PMCID: 3465621.
4. Nath K, Nelson DS, Putt ME, et al. Comparison of the Lonidamine Potentiated Effect of Nitrogen Mustard Alkylating Agents on the Systemic Treatment of DB-1 Human Melanoma Xenografts in Mice. PLoS One. 2016;11(6):e0157125.
5. Teicher BA, Holden SA, Ara G, et al. Whole-body hyperthermia and lonidamine as adjuvant therapy to treatment with cisplatin with or without local radiation in mouse bearing the lewis lung-carcinoma. International Journal of Hyperthermia. 1995;11(5):637-45.
6. Kim JH, Alfieri A, Kim SH, et al. Radiosensitization of meth-a fibro-sarcoma in mice by lonidamine. Oncology. 1984;41:36-8.
7. Golding JP, Wardhaugh T, Patrick L, et al. Targeting tumour energy metabolism potentiates the cytotoxicity of 5-aminolevulinic acid photodynamic therapy. Br J Cancer. 2013;109(4):976-82.
8. Guo L, Shestov AA, Worth AJ, et al. Inhibition of mitochondrial complex II by the anti-cancer agent lonidamine. J Biol Chem. 2016;291(1):42-57.
9. Nancolas B, Guo L, Zhou R, et al. The anti-tumour agent lonidamine is a potent inhibitor of the mitochondrial pyruvate carrier and plasma membrane monocarboxylate transporters. Biochem J. 2016 Apr 1;473(7):929-36.
Figures