5590
Online quantification of lactate concentration in microdialysate during cerebral activation using 1H-MRS and sensitive NMR microcoil1CRMSB, Université de Bordeaux, Bordeaux, France, 2Institut des Sciences Moléculaires, Université de Bordeaux, Bordeaux, France
Synopsis
The role of lactate in neuronal activation is central in the hypothesis of the astrocyte-to-neurons lactate shuttle. In this work, we implement highly sensitive 1H-MRS on brain microdialysate in order to monitor online the lactate fluctuations during neuronal activation in the S1BF area. The custom-made microcoil used in this study was shown to be sensitive enough for measuring a 40% increase in lactate concentration during brain stimulation.
Purpose
Often considered as a metabolic end product, lactate plays actually a substantial role in the brain, and in the last 20 years, a large number of studies revealed the use of lactate as a neuronal energetic substrate (1,2). Functional 1H MRS (fMRS) has been proposed for assessing non-invasively the temporal variations of lactate concentration during neuronal activation (3,4). However, the low physiological lactate concentration observed in-vivo (1-3mM) and its NMR methyl peak (1.3 ppm) overlapping with macromolecules and lipids peaks impede the detection of this metabolite by 1H fMRS. Despite reliability of metabolite quantification attainable at ultra-high fields, the temporal and spatial resolution of fMRS do not allow quantification of small (20-30%) lactate variation (4,5) expected during brain stimulation. In this study, we combined 1H MRS, microdialysis and home-built sensitive NMR microcoil to assess online, during neuronal activation, the lactate concentration of microliter dialysate from the stimulated area.Methods
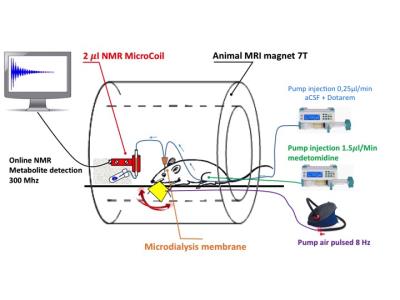
48h before MRS study, two microdialysis cannulae were implanted stereotaxically in the left and the right barrel cortex (S1BF area) of male Wistar rats (200g). The day of experiment, animals were anesthetized with i.v perfusion of metedomidine (100μg/kg/h). The microdialysis probes (2 mm long, 6 kD cutoff) were perfused at a rate of 0.25 µl/min with a solution of artificial cerebrospinal fluid, containing 1.5 mM Gd-DOTA (Dotarem®). MRI and MRS acquisitions were performed at 7 T (Bruker, Biospec 70/20). A proton surface coil was used to control microdialysis probe location in the S1BF area and the release of the perfusate into the brain. Then, the outlet tubing of the microdialysis probe was passed through a custom-made microsolenoidal coil (6) used for MRS acquisitions (see experimental set-up in Fig. 1). Spectra were obtained using a non-selective RF excitation (TR=2500 ms, averages= 100, scan time 4 minutes). After stabilization of microdialysis probe (1h), acquisitions were alternatively done during rest or activated period (30min each). Activation of the left barrel cortex was obtained by the stimulation of right vibrissae of the animals with an amagnetic pulsed air system (8Hz, 20s activated/20s rest).Results
The microdialysis probe
and its positioning in the S1BF area were accurately visualized using a 1H
surface coil.
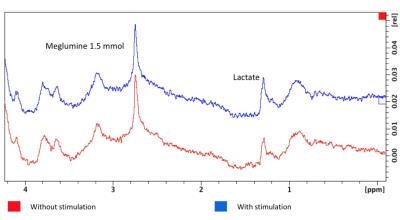
Exemplary spectra obtained during rest
period and activation are shown in Fig. 2. Lactate (methyl proton at 1.3 ppm),
meglumine the Dotarem® excipient (CH protons at 2.8 ppm) are readily observable
in each spectrum with SNR values above 20
for reference meglumine peak. Based on their amplitudes relative to the
meglumine peak, the upper limit for lactate concentration in the dialysate was
evaluated to be equal to 0.23 mM and 0.37 mM during rest and activation period,
respectively, corresponding to a 47 % increase of lactate concentration during
neuronal activation in the barrel cortex.Discussion
Taking advantages of microdialysis probe (removal of macromolecules and lipids NMR signals) and of highly sensitive NMR microcoil, we demonstrate the feasibility of detecting physiological variation in lactate concentration during neuronal activation using in vivo NMR spectroscopy. The observed variations of lactate concentration are in line with previously reported variations of lactate using microdialysis sampling and enzymatic assay of lactate (7). Beside its high detection sensitivity, the NMR detection coil was designed for minimal connecting and dead volumes between microdialysis membrane and the NMR microsolenoid. As a result, the variations of lactate amplitudes were monitored with a temporal resolution compatible with the rest and activation periods used in the experimental set-up.Conclusion
This new approach of microdialysis combined to sensitive real-time MRS acquisitions represent original and powerful approaches for elucidating the role of the astrocyte-to-neurons lactate shuttle in neuronal activation. Besides sampling of the extracellular fluid, the microdialysis probe could be used as well for local administration of, for instance, pharmacologic agents able to specifically block key lactate transporter.Acknowledgements
The authors acknowledge funding from the ANR program Innes and from the Laboratory of Excellence TRAIL ANR-10-LABX-57.References
1. Pellerin L et al., Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 2007;55:1251-1262.
2. Sampol et al., Glucose and lactate metabolism in the awake and stimulated rat: a 13C-NMR study. Front Neuroenergetics 2013; 5: 5.
3. Mangia et al., Dynamics of Lactate Concentration and Blood Oxygen Level-Dependent Effect in the Human Visual Cortex During Repeated Identical Stimuli. J. Neurosci. Research 2007 ; 85:3340–3346.
4. Mangia et al., The in vivo neuron-to-astrocyte lactate shuttle in human brain evidence from modeling of measured lactate levels during visual stimulation. J Neurochem. 2009;109(Suppl 1): 55–62.
5. Bednarík et al. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 2015;35(4):601-10.
6. Glöggler S et al. In vivo online magnetic resonance quantification of absolute metabolite concentrations in microdialysate. Sci Rep. 2016 Nov 4;6:36080.
7. Caesar et al. Glutamate receptor-dependent increments in lactate, glucose and oxygen metabolism evoked in rat cerebellum in vivo. J Physiol 2008; 586(5):337–1349.