5589
Relationship Between BOLD fMRI and Functional MRS the Medial Temporal Lobe1Department of Neurobiology and Behavior, University of California, UCI, Irvine, CA, United States, 2Center for the Neurobiology of Learning and Memory, University of California, Irvine, CA, United States
Synopsis
Structures in the medial temporal lobe (MTL) like the hippocampus play a critical role in memory. Functional disruptions of the MTL (typically studied with BOLD fMRI) are present in a range of disorders and diseases. The relative and indirect nature of BOLD makes it difficult to interpret findings such as the hippocampal “hyperactivity” that has been tied to age-related cognitive decline and the progression to Alzheimer’s Disease. In this work, “functional” MR spectroscopy is combined with simultaneous BOLD during memory tasks to investigate the relationship between the two. While task-related activity is observed in both, there are points of departure.
Background and Purpose
There is a great deal of evidence to suggest structural and functional disruptions of the MTL in both healthy and pathological aging and there is a great deal of research that attempts to use these as biomarkers for disease. The vast majority of the functional work has used BOLD fMRI. However, age-related (and disease-related) changes in the vasculature or vascular coupling have been shown to be present in BOLD and to account for results that would typically be attributed to age-related cognitive changes (1). This significantly limits our ability to understand the neural bases of the behavioral changes as observed effects may be purely artefactual and true effects may be obscured. Functional MRS (2, 3-5) is a novel technique that has the potential to address many of BOLD’s limitations by quantifying the levels of various metabolites in the brain that may be direct products of neuronal activity or markers of cellular loss/damage. This can give us a clearer link to the underlying neurobiology that is vital for any attempts at targeted therapy. Here, we combine functional MR spectroscopy (fMRS) with simultaneous BOLD in an effort to address this limitation. We extend existing fMRS work to studies of the hippocampus and parahippocampal gyrus, using a memory paradigm known to elicit strong BOLD fMRI signals in each (2).Methods
Our final experimental design will include three groups of participants (young, healthy aging, aMCI; n=24 per group) will participate in an initial consent and behavioral/neuropsychological testing session and a 1.5 hour MRI session. Preliminary results are presented from three healthy volunteers. Each session began with standard localizer scans and a 3D MP-RAGE structural scan (0.75 mm isotropic) and high resolution T2 weighted images, used to locate the regions of interest. Water suppressed and unsuppressed MRS acquisitions were acquired in an interleaved fashion with a TR 4s (2s each). Two dummy scans (8s total) preceded the dynamic 120 acquisitions to allow for steady state acquisition. The experimental paradigm was synchronized to start at the 3rd signal acquisition. The system-supplied shim was used to achieve a spectral width of less then 10 Hz. A blocked design was chosen to maximize SNR in both the fMRS and BOLD signals. Blocks were 30 s in duration and consisted of rest, novel scenes, familiar scenes, and a non-mnemonic perceptual baseline task and based on prior BOLD fMRI studies of the MTL (6). Each condition was presented 4 times corresponding to 2 minutes (30 signal averages each). Spectra were acquired using a single voxel PRESS sequence (7) in the left hippocampus (2.5 x 1.5 x 1.2 cm) and in the left parahippocampal cortex (4 x 2 x 1.5 cm), TE=35 ms, 2048 data points. The water suppressed and unsuppressed signals for each condition were averaged and spectral fitting was achieved through Tarquin (v.4.3.6) to quantify total NAA, total Cr and Glx. The unsuppressed water signal was decomposed into T2* through exponential fitting. This temporal T2* signal was detrended by removing baseline and linear drift and converted to percent signal change to compare across subjects.Results and Discussion
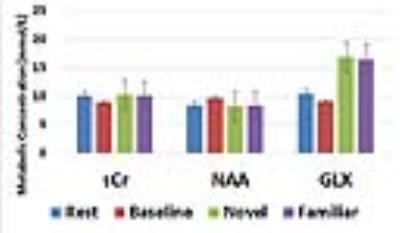
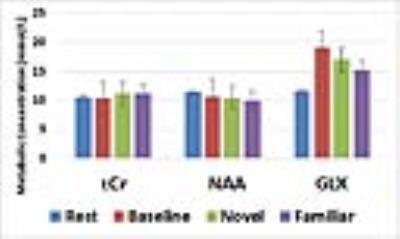
When collecting MRS data, the water unsuppressed 1H MRS signal was used as a proxy for BOLD fMRI (8-11). The mean value from each condition is reported as a % signal change from the mean in Table 1. Despite the small sample size, these BOLD effects replicate our prior work (6). Novel pictures elicited the highest level of response and familiar pictures elicited less than novel, but more than the perceptual baseline task in both the hippocampus and parahippocampal cortex. Thus, our proxy for BOLD is consistent with prior results using more typical BOLD fMRI scans. The hippocampal and parahippocampal metabolite concentrations are shown in Figures 1 and 2. NAA and Cr concentrations serve as a negative control and, as expected, showed no task modulation. Glx concentrations showed clear task modulations, demonstrating a reliable functional MRS signal. In both regions, novel and familiar pictures elicited higher levels of Glx than rest. In the hippocampus, the perceptual baseline was associated with very low levels of Glx while in the parahippocampal cortex, Glx levels were elevated. Thus, in both BOLD and Glx, novel and familiar pictures elevated their respective measures of activity. Yet, clear differences emerged. In particular, our two control conditions (simple rest and perceptual baseline) differed dramatically in the parahippocampal cortex (highest levels of Glx vs. lowest levels of BOLD). Therefore, even in a healthy, young population, there are both clear points of contact between these two measures of activity and clear points of departure.
Acknowledgements
Funding for this project was provided in part by a grant from the National Institutes on Aging R21-AG054092-01.References
1. D’Esposito, M., Deouell, L. Y. & Gazzaley, A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci 4, 863–72 (2003). 2. Apsvalka, D., Gadie, A., Clemence, M. & Mullins, P. G. Event-related dynamics of glutamate and BOLD effects measured using functional magnetic resonance spectroscopy (fMRS) at 3T in a repetition suppression paradigm. Neuroimage 118, 292-300 (2015). 3. Cleve, M., Gussew, A. & Reichenbach, J. R. In vivo detection of acute pain-induced changes of GABA+ and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. Neuroimage 105, 67-75 (2015). 4. Gussew, A. et al. Time-resolved functional 1H MR spectroscopic detection of glutamate concentration changes in the brain during acute heat pain stimulation. Neuroimage 49, 1895-1902 (2010). 5. Lally, N. et al. Glutamatergic correlates of gamma-band oscillatory activity during cognition: a concurrent ER-MRS and EEG study. Neuroimage 85 Pt 2, 823-833 (2014). 6. Stark, C. E. L. & Squire, L. R. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences 98, 12760–12766 (2001). 7. Bottomley, P. A. Selective volume method for performing localized NMR spectroscopy. United States of America, (1984). 8. Frahm, J., Kruger, G., Merboldt, K. D. & Kleinschmidt, A. Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med 35, 143-148 (1996). 9. Hennig, J. Functional spectroscopy to no-gradient fMRI. Neuroimage 62, 693-698 (2012). 10. Koush, Y., Elliott, M. A. & Mathiak, K. Single Voxel Proton Spectroscopy for Neurofeedback at 7 Tesla. Materials (Basel) 4, (2011). 11. Zhu, X. H. & Chen, W. Observed BOLD effects on cerebral metabolite resonances in human visual cortex during visual stimulation: a functional (1)H MRS study at 4 T. Magn Reson Med 46, 841-847 (2001).Figures