5584
Safety and Function of Programmable Ventriculo-Peritoneal Shunt Valves: An in vitro 7 Tesla Magnetic Resonance Imaging Study1Erwin L. Hahn Institute for MRI, University of Duisburg-Essen, Essen, Germany, 2Department of Neurosurgery, University Hospital Essen, University of Duisburg-Essen, Essen, Germany, 3High Field and Hybrid MR Imaging, University Hospital Essen, University of Duisburg-Essen, Essen, Germany
Synopsis
This in vitro study tests function, safety and image artifacts of the two worldwide most frequently implanted programmable VP-shunt valves in a 7 Tesla whole body MRI system. Both tested programmable VP-shunt valves lost their ability to be reprogramed after exposure to the static magnetic field and are therefore unsafe for use in 7 Tesla whole body scanners in their current design. Magnetic coercivity of the permanent magnets in the programming mechanisms was insufficient. Image artifacts adjacent to the valves, however, were tolerable.
Introduction
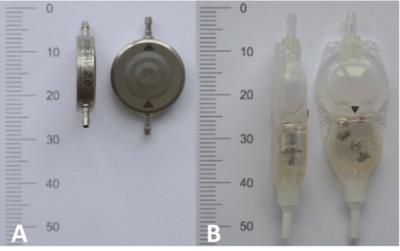
In the last years, the quantity of ultra-high field (UHF) magnetic resonance imaging (MRI) studies demonstrating diagnostic benefits in neuro imaging has increased rapidly [1]. Today, MRI is the primary diagnostic tool in neurosurgical patients and mandatory for follow-up of the majority of these patients after surgical treatment. The most commonly used neurosurgical implants are programmable ventriculo-peritoneal (VP) shunts. This in vitro study tests function, safety and image artifacts of the two worldwide most frequently implanted programmable VP-shunt valves (Figure 1) in a 7T whole body MRI system.Material and Methods
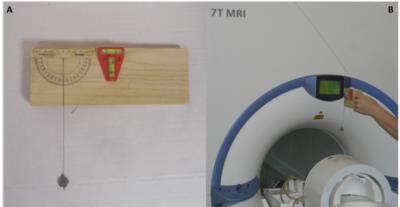
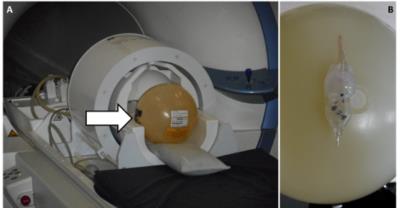
All tests were performed using a passively shielded whole-body MRI system (Magnetom 7T; Siemens Healthcare, Germany) equipped with a 1/32-channel Tx/Rx head radiofrequency (RF) coil (Nova Medical, MA). Three proGAV 2.0 programmable VP-shunt valves (Miethke GmbH, Germany) and 3 CODMAN CERTAS® Plus programmable VP-shunt valves (Codman & Shurtleff, Inc., MA), all certified as 3T MR conditional, were tested in a three-step procedure. 1) Deflection angle tests [2] were performed at the location of the highest static magnetic field gradient (5 T/m) close to the scanner opening (Figure 2). 2) The valves were fixed on a standard spherical phantom and tested for keeping the programmed pressure setting and ability to be reprogramed. The phantom with the valve was positioned in the head coil (Figure 3) and centered in the magnet’s isocenter using a motorized patient table with a constant speed of 10 cm/s. Then the phantom was removed from the scanner and the valve was tested for keeping the programmed pressure setting and ability to be reprogramed using the vendor provided tools. This procedure was repeated 3 times for all valves and for the following positions on the phantom:1. strictly lateral; 2. strictly cranial, 3. cranial with 22.5° tilt anteriorly. 3) The valves were fixed on a spherical phantom and positioned strictly right lateral in the head coil. Scans were performed for both VP shunt models separately. Acquired sequences included MPRAGE, as well as gradient and spin echo sequences according to [3].Results
1) Deflection angles were moderate (13°, 14°, 13°) for the proGAV 2.0 programmable VP-shunt valves and nearly critical (43°, 43°, 41°) for the CODMAN CERTAS® Plus programmable VP-shunt valves.
2) The proGAV 2.0 programmable VP-shunt valves kept the programmed pressure settings with ability to be reprogramed in position 1 and 2 on the phantom. The valves kept their pressure setting, but ability to be reprogramed was lost after the first test with position 3 on the phantom. A magnetometer showed reversed polarity of the permanent magnets that are the crucial part of the programming mechanism within the valves. The CODMAN CERTAS® Plus programmable VP-shunt valves changed their pressure setting and ability to be reprogramed was lost after the first test in position 1, 2 and 3 on the phantom, respectively. A magnetometer also showed reversed polarity of the permanent magnets that are the crucial part of the programming mechanism within the valves.
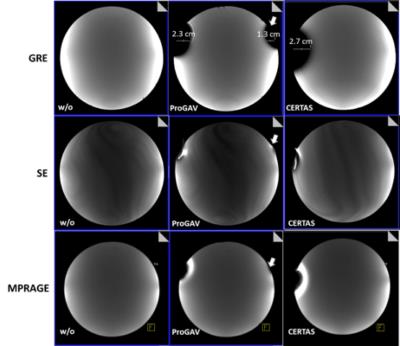
3) The image signal homogeneity was unaltered by the shunt valves in the center of the phantom and image artifacts adjacent to the valves were tolerable. Figure 4 illustrates image homogeneity and artifacts for both valve systems and different MR sequences.
Discussion and Conclusion
The proGAV 2.0 programmable VP-shunt valves showed a moderate deflection in the static magnetic field of the scanner that can be considered conditional for a neurosurgical implant. The nearly critical deflection of the CODMAN CERTAS® Plus programmable VP-shunt valves was probably caused by the larger permanent magnets used in the programming mechanism and they should be treated with caution for an exposure to a 7T system. In particular, the magnetic field gradient reaches values of 7 T/m at locations accessible to the patient within an actively shielded 7T MR system. [4] All shunt valves lost their ability to be reprogramed after a series of exposures to the static magnetic field. The insufficient magnetic coercivity of the permanent magnets used in the programming mechanisms renders the valves unusable after exposure to the static magnetic field of a 7T MR system. Permanent magnets with higher magnetic coercivity should help to overcome this issue. The image signal homogeneity was unaltered by the shunt valves and image artifacts adjacent to the valves were tolerable. In conclusion, both tested programmable VP-shunt valves are unsafe for use in 7T systems in their current form. Altered programming mechanisms using permanent magnets with sufficient magnetic coercivity may allow development of programmable VP-shunt valves that are conditional (providing conditionality regarding RF induced heating) for use in 7T MR systems.Acknowledgements
The research leading to these results has received funding from the Interne Forschungsförderung Essen (IFORES), University Hospital Essen, University Duisburg-Essen.References
[1] Kraff O, et al., MRI at 7 Tesla and above: demonstrated and potential capabilities. J Magn Reson Imaging 2015;41(1):13-33.
[2] ASTM, Standard Test Method for Measurement of Magnetically Induced Displacement Force on Medical Devices in the Magnetic Resonance Environment, in F2052 (2015).
[3] ASTM, Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants, in F2119 (2013).
[4] Siemens Healthcare GmbH, Magnetom 7T System Owner Manual, MR compatibility data sheet, version 01/13, VB17A 3rd edition.
Figures